Science 10 Chapter 4 3 Balancing Chemical Equations
Science 10 Chapter 4. 3 Balancing Chemical Equations

Today q Learning check q Review chemical compounds and formulas q Chemical Reactions!!!
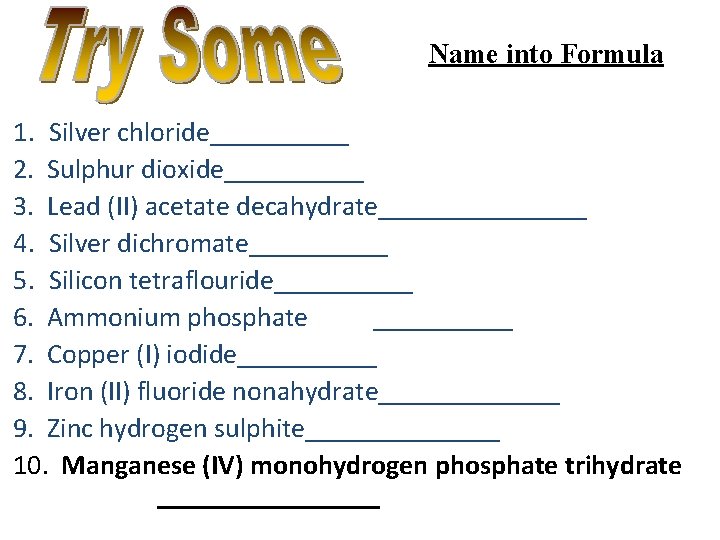
Name into Formula 1. Silver chloride_____ 2. Sulphur dioxide_____ 3. Lead (II) acetate decahydrate________ 4. Silver dichromate_____ 5. Silicon tetraflouride_____ 6. Ammonium phosphate _____ 7. Copper (I) iodide_____ 8. Iron (II) fluoride nonahydrate_______ 9. Zinc hydrogen sulphite_______ 10. Manganese (IV) monohydrogen phosphate trihydrate ________

Chemical Changes • Nothing is created or destroyed, only rearranged • Reactants = products • 200 yrs ago John Dalton realized atoms rearrange • # of each atom in reactants = # of each atom in products

The LAW: Conservation of mass • Antoine and Marie Anne Lavoisier in the 1700’s • Atoms are neither created or destroyed in chemical reactions • Mass reactants = mass products

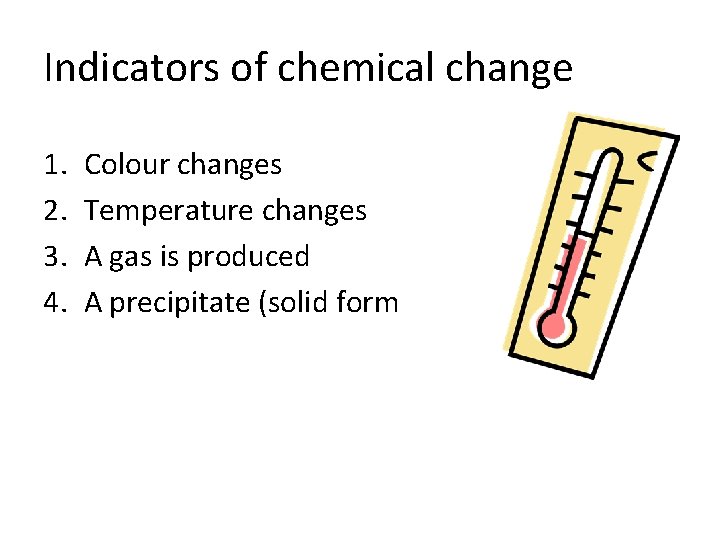
Indicators of chemical change 1. 2. 3. 4. Colour changes Temperature changes A gas is produced A precipitate (solid form
Chemical Reactions • Occur when new substances are created reactants products Can be written as: • A word equation: nitrogen monoxide + oxygen nitrogen dioxide • A symbolic equation: 2 NO(g) + O 2(g) 2 NO 2(g)
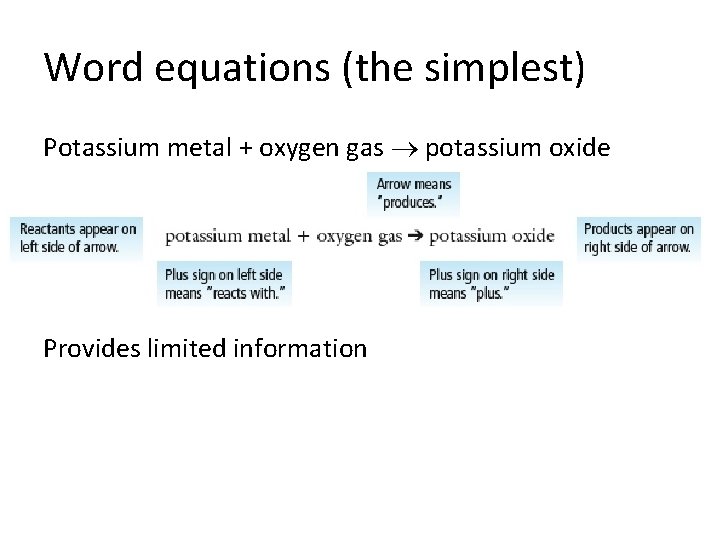
Word equations (the simplest) Potassium metal + oxygen gas potassium oxide Provides limited information
Skeletal Equations (Symbolic) • Show formulas of compounds/elements, but not quantities of atoms e. g. , K + O 2 K 2 O
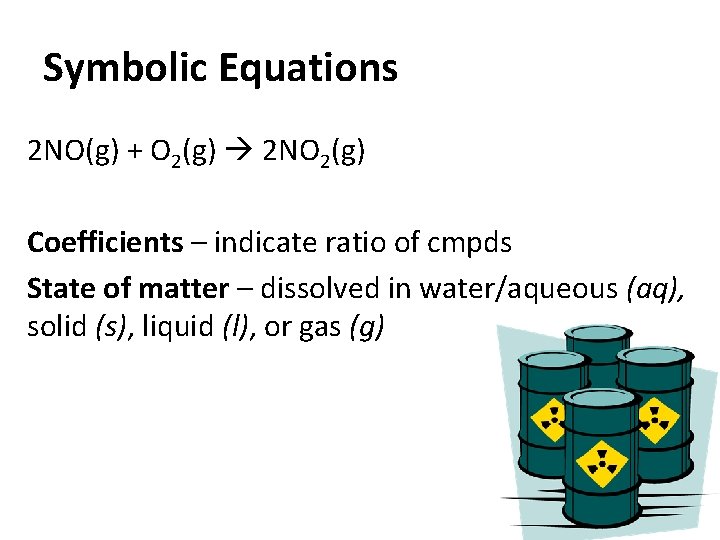
Symbolic Equations 2 NO(g) + O 2(g) 2 NO 2(g) Coefficients – indicate ratio of cmpds State of matter – dissolved in water/aqueous (aq), solid (s), liquid (l), or gas (g)
Balanced chemical equation • Shows all atoms and their quantities • Number of each atom should be equal on both sides of the reaction arrow • Always use smallest whole number ratio • To balance, change coefficients, never subscripts e. g. , 4 K + O 2 2 K 2 O Hg. O Hg + O 2
Helpful hints 1. 2. 3. 4. Balance metals first Count polyatomic groups as 1 atom Balance oxygen atoms last Odd/even problem? Double it! ____Mg + ____HCl → ____Mg. Cl 2 + ____H 2 + ____ N 2 → ____ NH 3 ____ Fe + ____ Br 2 → ____ Fe. Br 3

____Sn(NO 2)4 ___K 3 PO 4 → ___KNO 2 ___Sn 3(PO 4)4 ____P 4 + ____I 2 → ____PI 3 ____Al + ____O 2 → ____Al 2 O 3
Today q Learning check q Review chemical compounds and formulas q Chemical Reactions #X#(s) A B q Due tomorrow: workbook pages 71 and 73 q Due Monday: workbook pages 77, (78 79 even #’s), 80
- Slides: 16