Schrodinger model for the atom 1926 the 3


- Slides: 12

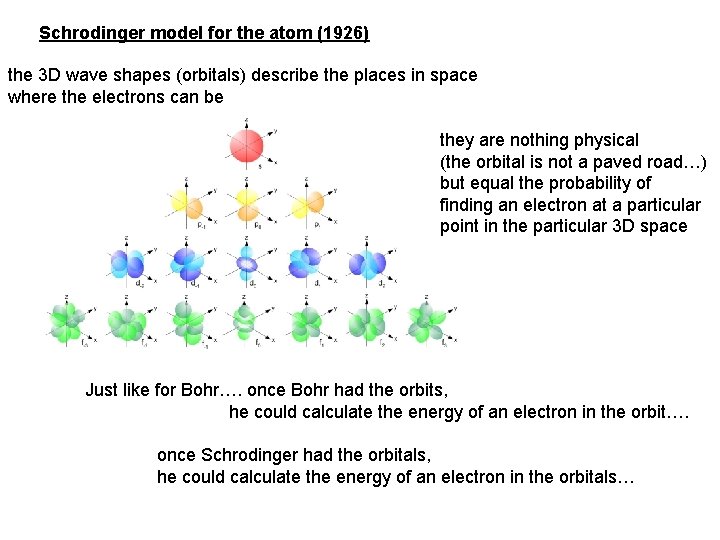
Schrodinger model for the atom (1926) the 3 D wave shapes (orbitals) describe the places in space where the electrons can be they are nothing physical (the orbital is not a paved road…) but equal the probability of finding an electron at a particular point in the particular 3 D space Just like for Bohr…. once Bohr had the orbits, he could calculate the energy of an electron in the orbit…. once Schrodinger had the orbitals, he could calculate the energy of an electron in the orbitals…

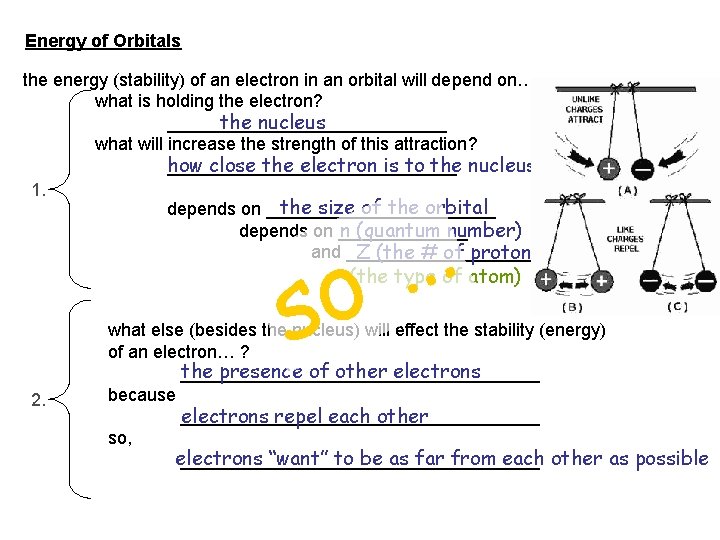
Energy of Orbitals the energy (stability) of an electron in an orbital will depend on… what is holding the electron? the nucleus ______________ what will increase the strength of this attraction? how close the electron is to the nucleus _______________ depends on ____________ the size of the orbital n (quantum number) depends on _______ and __________ Z (the # of protons) (the type of atom) as n goes ___ UP the size of the orbital ______ goes UP on average the electron is further ____ from the nucleus so, _____ stable less _____ higher in energy

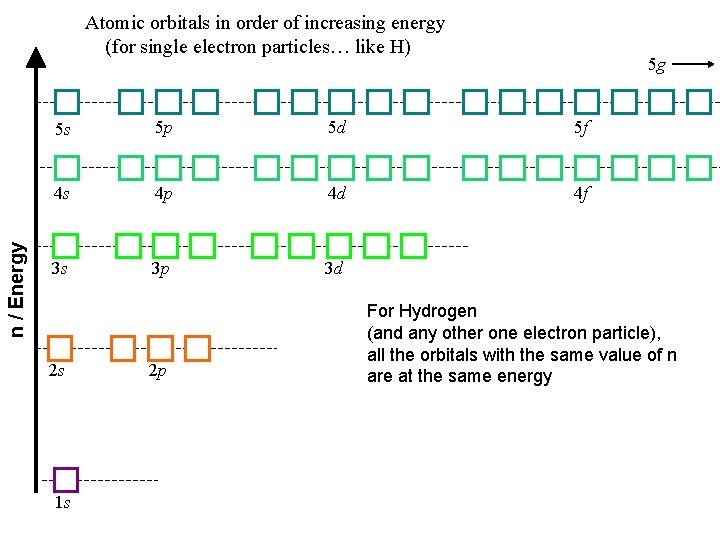
n / Energy Atomic orbitals in order of increasing energy (for single electron particles… like H) 5 g 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 1 s 2 p For Hydrogen (and any other one electron particle), all the orbitals with the same value of n are at the same energy

Ground state electron configurations of atoms of the elements distribution of the e- of an atom in the orbitals (arrangement) Most stable NOTE, the e- can be in any of the infinite # of orbitals, but one arrangement will be the most stable – ground state Electrons as close to the nucleus as possible Aufbau Principle - electrons will occupy the lowest energy (closest to the nucleus) orbital available

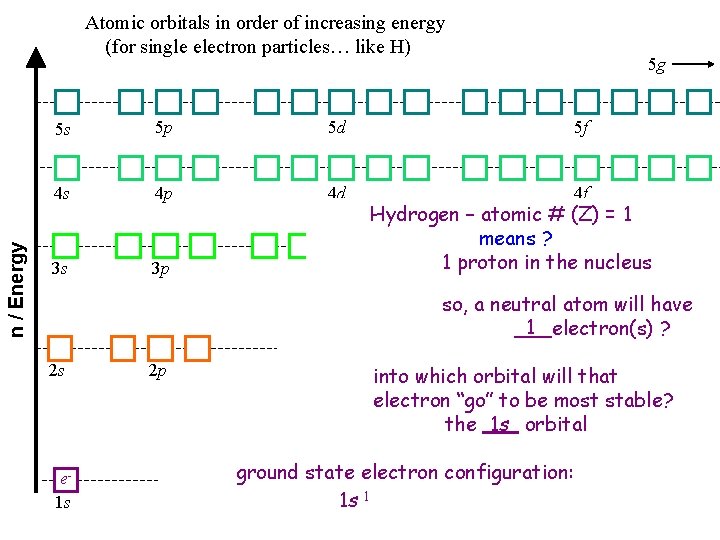
n / Energy Atomic orbitals in order of increasing energy (for single electron particles… like H) 5 g 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d Hydrogen – atomic # (Z) = 1 means ? 1 proton in the nucleus so, a neutral atom will have 1 ___electron(s) ? 2 s e 1 s 2 p into which orbital will that electron “go” to be most stable? the 1 s orbital ground state electron configuration: 1 s 1

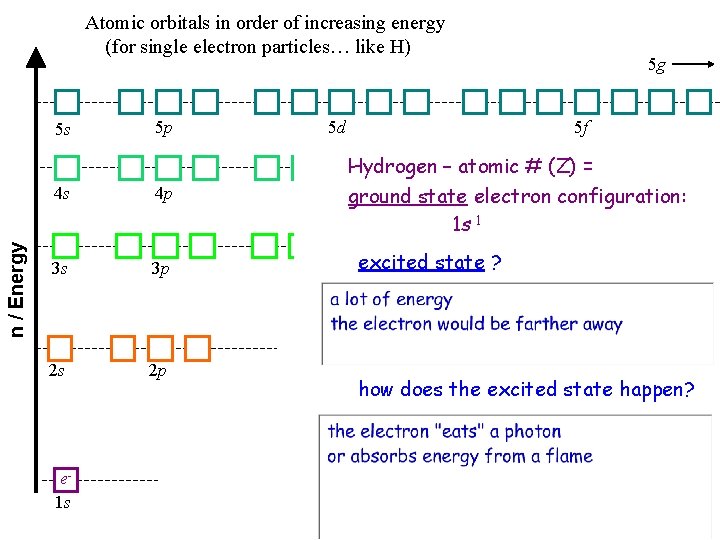
Atomic orbitals in order of increasing energy (for single electron particles… like H) 5 s 5 p 5 g 5 d 5 f Hydrogen – atomic # (Z) = 4 s 4 p 4 d ground state electron configuration: 4 f n / Energy 1 s 1 3 s 3 p 2 s 2 p e 1 s 3 d excited state ? how does the excited state happen?

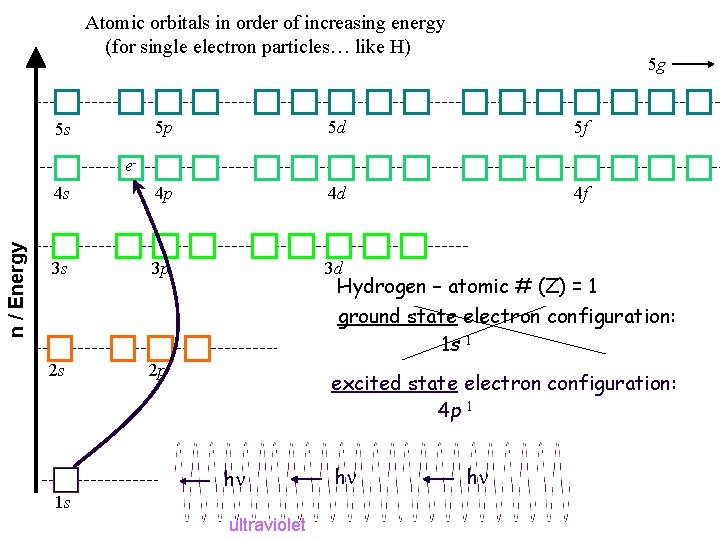
Atomic orbitals in order of increasing energy (for single electron particles… like H) 5 g 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d 5 s n / Energy e- Hydrogen – atomic # (Z) = 1 ground state electron configuration: 1 s 1 2 s e 1 s 2 p excited state electron configuration: 4 p 1 h ultraviolet h h

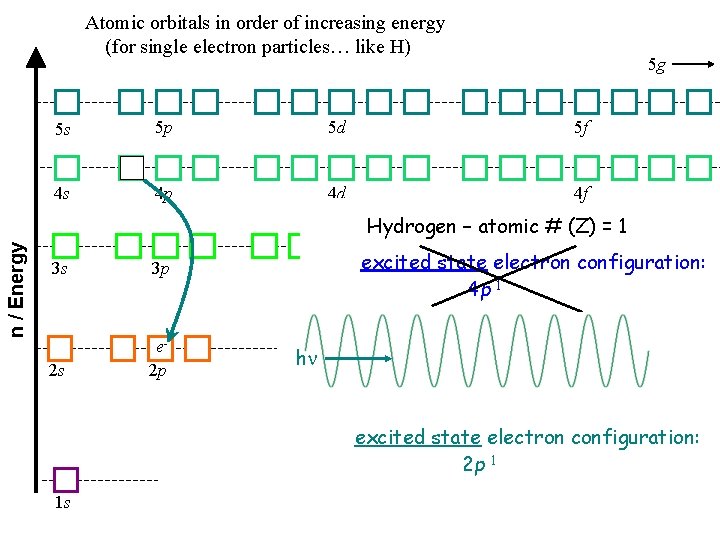
Atomic orbitals in order of increasing energy (for single electron particles… like H) 5 s 5 g 5 p 5 d 5 f 4 p 4 d 4 f e 4 s n / Energy Hydrogen – atomic # (Z) = 1 3 s 3 p 2 s e 2 p 3 d excited state electron configuration: 4 p 1 h excited state electron configuration: 2 p 1 1 s

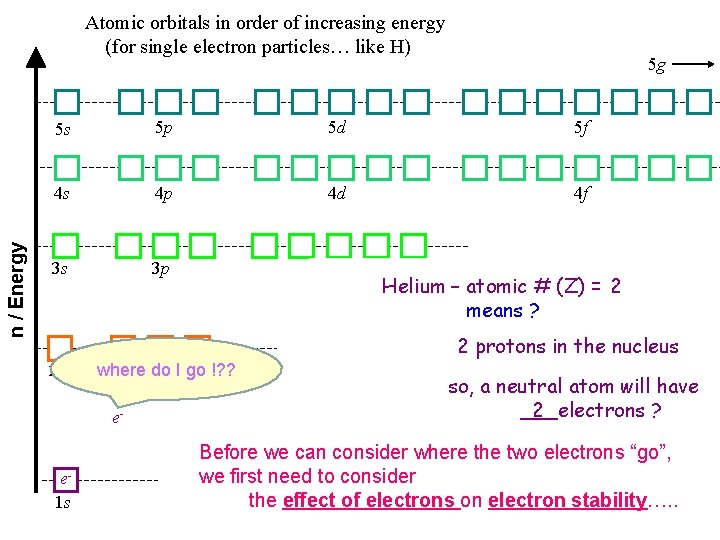
n / Energy Atomic orbitals in order of increasing energy (for single electron particles… like H) 5 g 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d Helium – atomic # (Z) = 2 means ? 2 protons in the nucleus 2 s where 2 p do I go !? ? e- e 1 s so, a neutral atom will have ___electrons ? 2 Before we can consider where the two electrons “go”, we first need to consider the effect of electrons on electron stability…. .

Energy of Orbitals the energy (stability) of an electron in an orbital will depend on… what is holding the electron? the nucleus ______________ what will increase the strength of this attraction? how close the electron is to the nucleus _______________ 1. the size of the orbital depends on ____________ n (quantum number) depends on _______ and __________ Z (the # of protons) … o s (the type of atom) 2. what else (besides the nucleus) will effect the stability (energy) of an electron… ? the presence of other electrons __________________ because __________________ electrons repel each other so, electrons “want” to be as far from each other as possible __________________

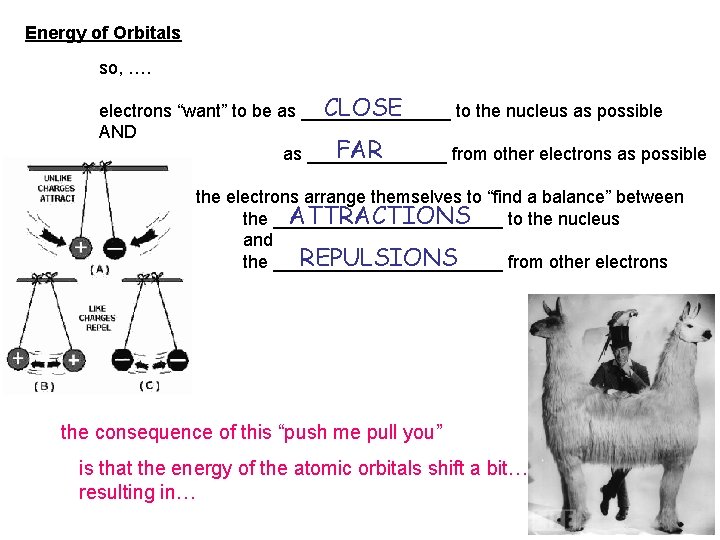
Energy of Orbitals so, …. CLOSE electrons “want” to be as ________ to the nucleus as possible AND FAR as _______ from other electrons as possible the electrons arrange themselves to “find a balance” between ATTRACTIONS the ____________ to the nucleus and REPULSIONS the ____________ from other electrons the consequence of this “push me pull you” is that the energy of the atomic orbitals shift a bit… resulting in…

on to electron_configurations_2