SCHEDULE II ATTENTIONDEFICIT HYPERACTIVITY DISORDER ADHD Sara J












































![FDA: THE BOXED WARNING Pediatric: [U. S. Boxed Warning]: Use with caution in pediatric FDA: THE BOXED WARNING Pediatric: [U. S. Boxed Warning]: Use with caution in pediatric](https://slidetodoc.com/presentation_image/1c765dd31a695a7d6cf422610f2071a2/image-78.jpg)






- Slides: 88

SCHEDULE II: ATTENTION-DEFICIT / HYPERACTIVITY DISORDER (ADHD) Sara J. D. Bork, Pharm. D, MBA April 14 th, 2016 PHARMACY

DISCLOSURES • Speaker Disclosures • Sara Bork does not have any actual or potential conflicts of interest in relation to this program • Editorial advisors and ACPE administrator disclosures • No actual or potential conflicts of interest in relation to this program are reported. PHARMACY

OBJECTIVES • Compare characteristics of Attention-Deficit/Hyperactivity Disorder in children, adolescents and adults • Provide an overview of the current American Academy of Pediatrics Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents and application in select clinical practice scenarios • Differentiate pharmacologic treatment options for Attention. Deficit/Hyperactivity Disorder in children, adolescents and adults PHARMACY

IN PREPARATION FOR TODAY… • • • Define Attention Deficit / Hyperactivity Disorders (ADHD) Prevalence of ADHD Differentiation of child, adolescent and adult ADHD AAP Practice Guidelines Medication Treatment • • PHARMACY Methylphenidate Dextroamphetamine Dexmethylphenidate Lisdexamfetamine Atomoxetine Clonidine Guanfacine

ATTENTION-DEFICIT / HYPERACTIVITY DISORDER (ADHD) PHARMACY

ADHD Attention-deficit / hyperactivity disorder (ADHD) is a disorder that manifests with persistent symptoms of inattention and/or hyperactivityimpulsivity. These symptoms affect academic, occupational, and social functioning. PHARMACY DSM-IV-TR. 4 th Edition, 2000.

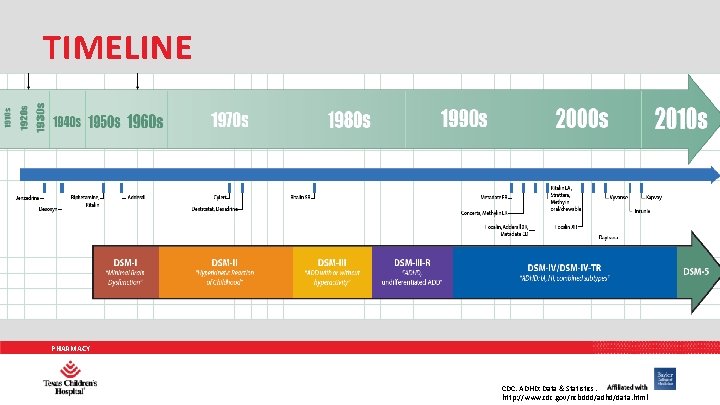
TIMELINE PHARMACY CDC. ADHD: Data & Statistics. http: //www. cdc. gov/ncbddd/adhd/data. html

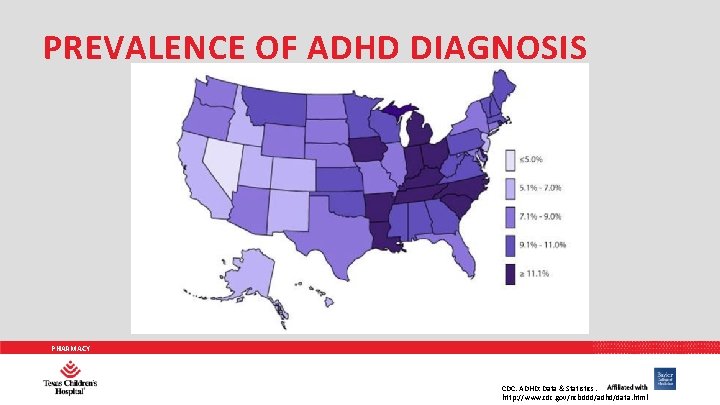
PREVALENCE OF ADHD • ADHD in children 5 – 17 years: 9% • ADHD in children 12 – 17 years: 3 million children • Males 12. 3%: Females 5. 5% • Studies of ADHD in childhood suggest persistence of ADHD • Into adolescence: 75% • Into adulthood: 50% PHARMACY Curr Opin Pediatr 2014; 26: 119 -129.

PREVALENCE OF ADHD DIAGNOSIS PHARMACY CDC. ADHD: Data & Statistics. http: //www. cdc. gov/ncbddd/adhd/data. html

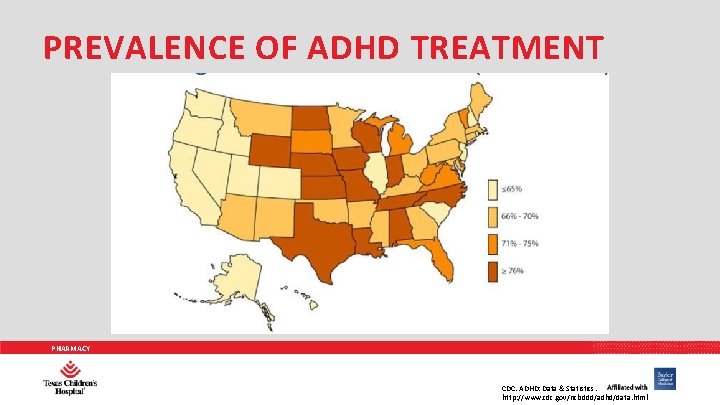
PREVALENCE OF ADHD TREATMENT PHARMACY CDC. ADHD: Data & Statistics. http: //www. cdc. gov/ncbddd/adhd/data. html

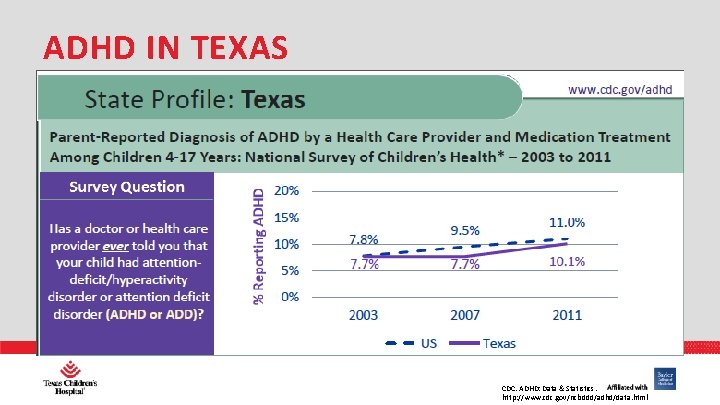
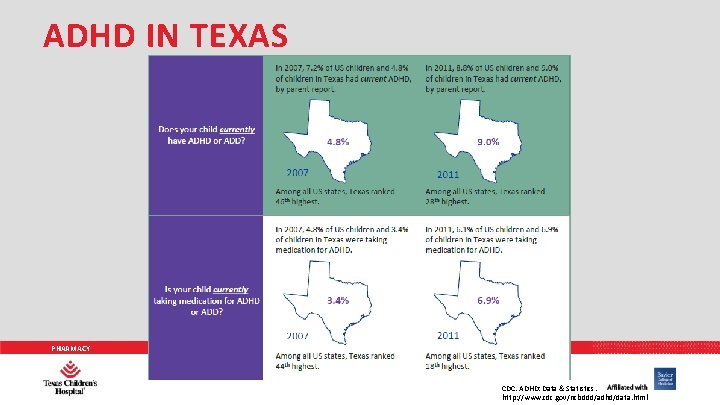
ADHD IN TEXAS PHARMACY CDC. ADHD: Data & Statistics. http: //www. cdc. gov/ncbddd/adhd/data. html

ADHD IN TEXAS PHARMACY CDC. ADHD: Data & Statistics. http: //www. cdc. gov/ncbddd/adhd/data. html

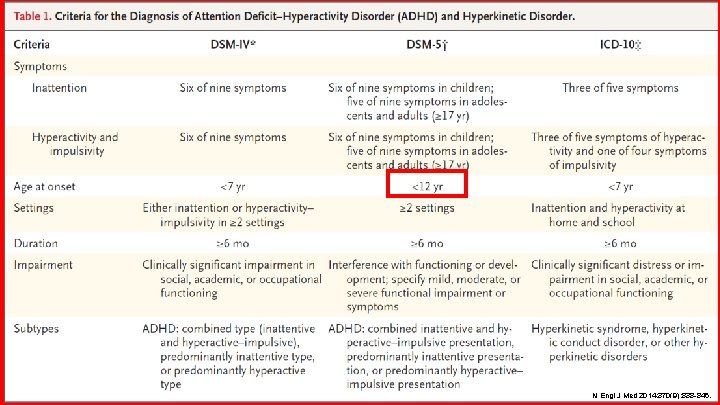
PHARMACY N Engl J Med 2014; 370(9): 838 -846.

PHARMACY N Engl J Med 2013; 369(20): 1935 -1944.

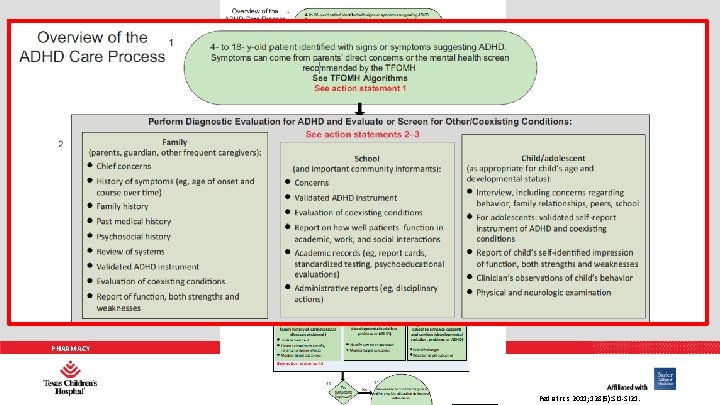
AAP PRACTICE GUIDELINES 1. The primary care clinician should initiate an evaluation for ADHD for any child 4 through 18 years of age who presents with academic or behavioral problems and symptoms of inattention, hyperactivity, or impulsivity (quality of evidence B/strong recommendation). PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

PHARMACY Pediatrics 2011; 128(5): SI 1 -SI 21.

ASSESSMENT OF ADHD PHARMACY J Am Acad Child Adolesc Psychiatry. 2007; 46(7): 894 -921.

AAP PRACTICE GUIDELINES 2. To make a diagnosis of ADHD, the primary care clinician should determine that Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria have been met (including documentation of impairment in more than 1 major setting); information should be obtained primarily from reports from parents or guardians, teachers, and other school and mental health clinicians involved in the child’s care. The primary care clinician should also rule out any alternative cause (quality of evidence B/strong recommendation). PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

PHARMACY N Engl J Med 2014; 370(9): 838 -846.

INATTENTION PHARMACY N Engl J Med 2013; 369(20): 1935 -1944.

HYPERACTIVITY / IMPULSIVITY PHARMACY N Engl J Med 2013; 369(20): 1935 -1944.

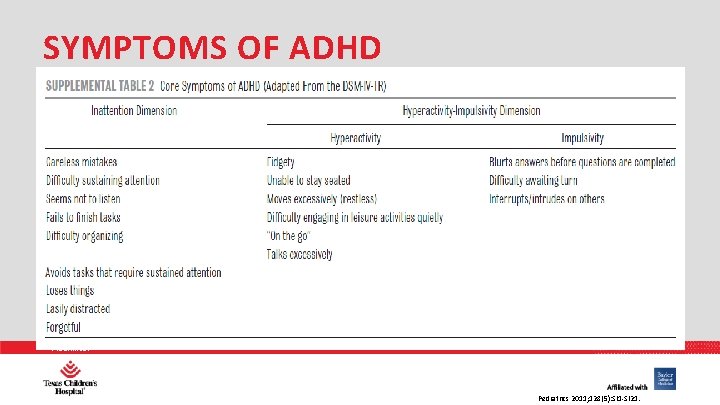
SYMPTOMS OF ADHD PHARMACY Pediatrics 2011; 128(5): SI 1 -SI 21.

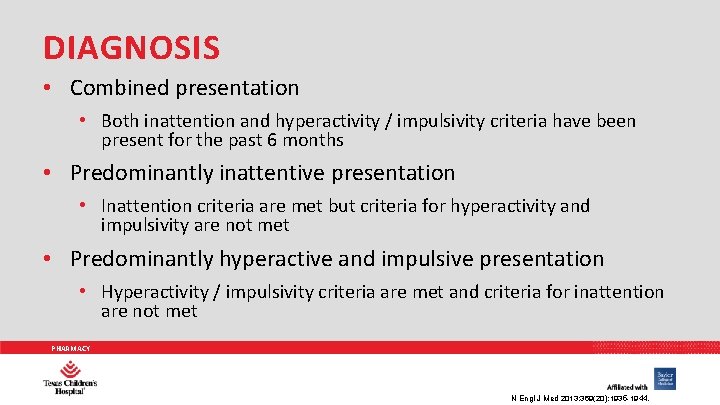
DIAGNOSIS • Combined presentation • Both inattention and hyperactivity / impulsivity criteria have been present for the past 6 months • Predominantly inattentive presentation • Inattention criteria are met but criteria for hyperactivity and impulsivity are not met • Predominantly hyperactive and impulsive presentation • Hyperactivity / impulsivity criteria are met and criteria for inattention are not met PHARMACY N Engl J Med 2013; 369(20): 1935 -1944.

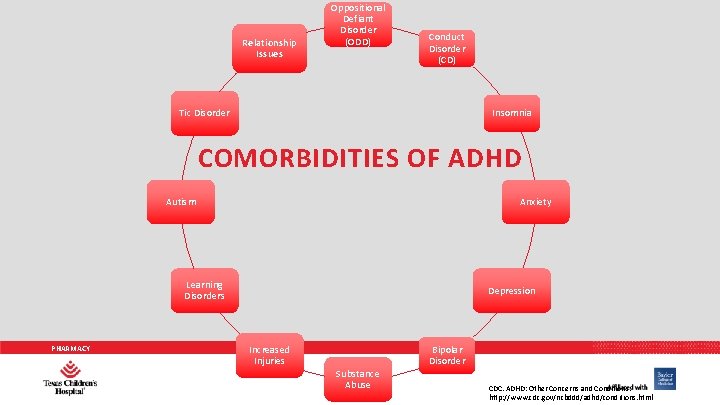
AAP PRACTICE GUIDELINES 3. In the evaluation of a child for ADHD, the primary care clinician should include assessment for other conditions that might coexist with ADHD, including emotional or behavioral (e. g. , anxiety, depressive, oppositional defiant, and conduct disorders), developmental (e. g. , learning and language disorders or other neurodevelopmental disorders), and physical (e. g. , tics, sleep apnea) conditions (quality of evidence B/strong recommendation). PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

Relationship Issues Oppositional Defiant Disorder (ODD) Conduct Disorder (CD) Insomnia Tic Disorder COMORBIDITIES OF ADHD Autism Anxiety Learning Disorders PHARMACY Depression Increased Injuries Substance Abuse Bipolar Disorder CDC. ADHD: Other Concerns and Conditions. http: //www. cdc. gov/ncbddd/adhd/conditions. html

AAP PRACTICE GUIDELINES 4. The primary care clinician should recognize ADHD as a chronic condition and, therefore, consider children and adolescents with ADHD as children and youth with special health care needs. Management of children and youth with special health care needs should follow the principles of the chronic care model and the medical home (quality of evidence B/strong recommendation). PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

PHARMACY Pediatrics 2011; 128(5): SI 1 -SI 21.

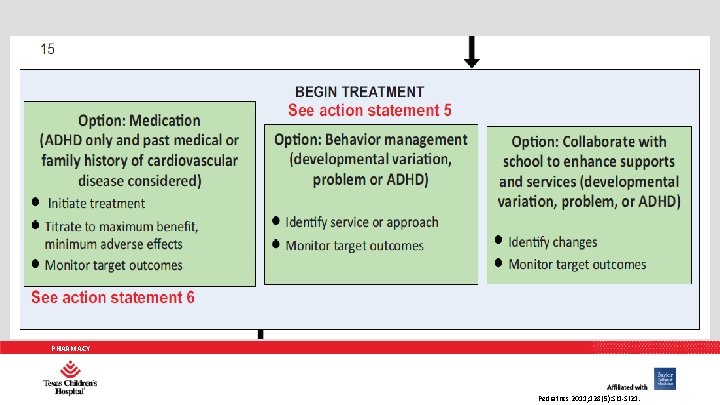
AAP PRACTICE GUIDELINES 5. Recommendations for treatment of children and youth with ADHD vary depending on the patient’s age: a. For preschool-aged children (4– 5 years of age), the primary care clinician should prescribe evidence-based parent- and/or teacher-administered behavior therapy as the first line of treatment (quality of evidence A/strong recommendation) PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

AAP PRACTICE GUIDELINES 5. Recommendations for treatment of children and youth with ADHD vary depending on the patient’s age: a. And may prescribe methylphenidate if the behavior interventions do not provide significant improvement and there is moderate-to severe continuing disturbance in the child’s function. In areas where evidencebased behavioral treatments are not available, the clinician needs to weigh the risks of starting medication at an early age against the harm of delaying diagnosis and treatment (quality of evidence B/recommendation). PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

AAP PRACTICE GUIDELINES 5. Recommendations for treatment of children and youth with ADHD vary depending on the patient’s age: b. For elementary school–aged children (6– 11 years of age), the primary care clinician should prescribe US Food and Drug Administration–approved medications for ADHD (quality of evidence A/strong recommendation) and/or evidence-based parent and/or teacher-administered behavior therapy as treatment for ADHD, preferably both (quality of evidence B/strong recommendation). PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

AAP PRACTICE GUIDELINES 5. Recommendations for treatment of children and youth with ADHD vary depending on the patient’s age: b. The evidence is particularly strong for stimulant medications and sufficient but less strong for atomoxetine, extended-release guanfacine, and extended-release clonidine (in that order) (quality of evidence A/strong recommendation). PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

AAP PRACTICE GUIDELINES 5. Recommendations for treatment of children and youth with ADHD vary depending on the patient’s age: c. For adolescents (12– 18 years of age), the primary care clinician should prescribe Food and Drug Administration– approved medications for ADHD with the assent of the adolescent (quality of evidence A/strong recommendation) and may prescribe behavior therapy as treatment for ADHD (quality of evidence C/recommendation), preferably both. PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

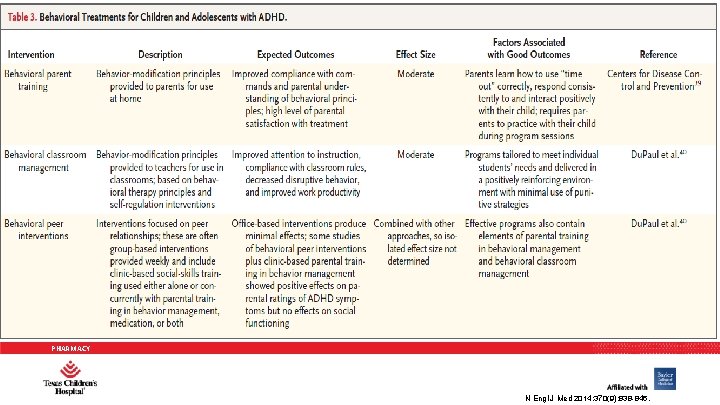
NONPHARMACOLOGIC OPTIONS • AAP guidelines recommend Behavioral Modification Training (BMT) as first line for children 4 -6 years • Combination of behavioral therapies with medication is considered most effective therapy for children > 6 years PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

PHARMACY N Engl J Med 2014; 370(9): 838 -846.

BEHAVIORAL PARENT TRAINING • 10 -20 weekly sessions (1 to 2 hours) during which time parents: 1. are given information about the nature of ADHD 2. learn to attend more carefully to their child’s misbehavior and to when their child complies 3. establish a home token economy 4. use time out effectively 5. manage noncompliant behaviors in public settings 6. use a daily school report card 7. anticipate future misconduct PHARMACY J Am Acad Child Adolesc Psychiatry. 2007; 46(7): 894 -928.

BEHAVIORAL THERAPY: AT HOME • • Create a routine Get organized Avoid distractions Limit choices Change your interactions with your child Use goals and rewards Discipline effectively Help your child discover a talent Patient & Family PHARMACY CDC. ADHD: Treatment. http: //www. cdc. gov/ncbddd/adhd/treatment. html

BEHAVIORAL THERAPY: IN THE CLASSROOM • • • Use a homework folder for communications Make assignments clear Give positive reinforcement Be sensitive to self-esteem issues Involve the school counselor or psychologist Patient & Family School PHARMACY CDC. ADHD: Treatment. http: //www. cdc. gov/ncbddd/adhd/treatment. html

BEHAVIORAL THERAPY: TECHNIQUES PHARMACY Pediatrics 2001: 108(4); 1033 -1044.

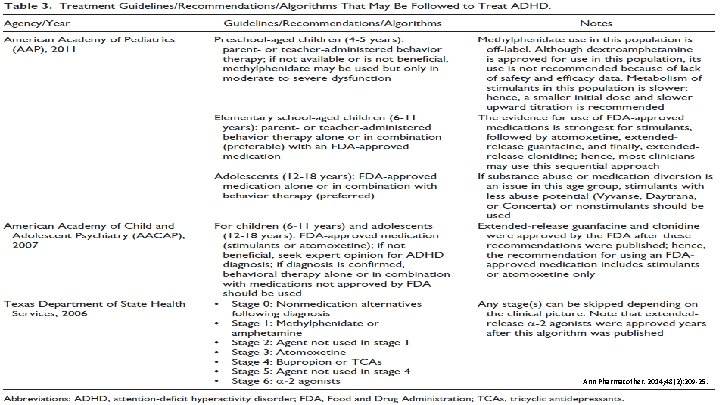
GUIDELINES FOR TREATMENT • The Texas Children's Medication Algorithm Project Revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. (2006) Patient & Family School • American Academy of Child Adolescent Psychiatry (2007) Health Care Professional • American Academy of Pediatrics (2011) All recommend a stimulant as first line PHARMACY J Am Acad Child Adolesc Psychiatry. 2006; 45(6): 642 -57. J Am Acad Child Adolesc Psychiatry. 2007; 46(7): 894 -928. Pediatrics 2011; 128(5): 1007 -1022.

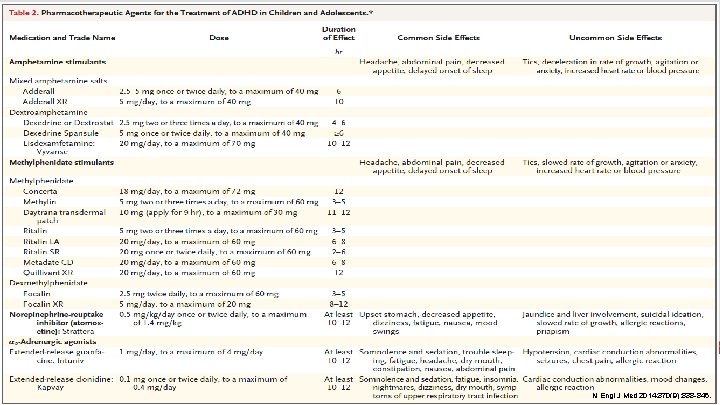
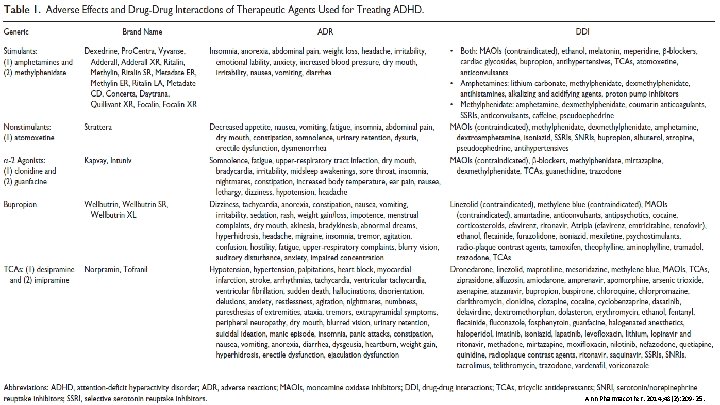
PHARMACY Ann Pharmacother. 2014; 48(2): 209 -25.

TEXAS CMAP ALGORITHM: COMORBIDITIES PHARMACY J Am Acad Child Adolesc Psychiatry. 2006 Jun; 45(6): 642 -57.

TREATMENT PHARMACY

TREATMENT: INDIVIDUALIZE THERAPY • • • Psychoeducation • Education on signs and symptoms of ADHD • Education on the misconceptions about ADHD and its treatment Psychotherapy • Behavioral parent therapy • Behavioral classroom management • Behavioral peer interventions Medications • Stimulants • Non-Stimulants PHARMACY N Engl J Med 2014; 370(9): 838 -846.

GOALS OF TREATMENT • Ensure safety of the patient • Develop a therapeutic relationship • Change the patient’s behavior / symptoms • • • Improve written and verbal communication Improve academic performance Decrease disruptive behaviors Improve self-esteem Reduce requirement of supervision Enhance safety PHARMACY Ped in Review. 2003: 24(3); 92 -98.

AAP PRACTICE GUIDELINES 6. The primary care clinician should titrate doses of medication for ADHD to achieve maximum benefit with minimum adverse effects (quality of evidence B/strong recommendation). PHARMACY Pediatrics 2011; 128(5): 1007 -1022.

PHARMACOLOGIC TREATMENT PHARMACY

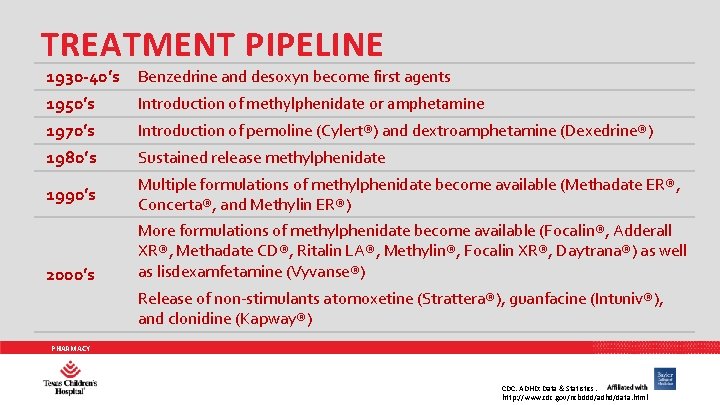
TREATMENT PIPELINE 1930 -40’s 1950’s 1970’s Benzedrine and desoxyn become first agents 1980’s Sustained release methylphenidate 1990’s Multiple formulations of methylphenidate become available (Methadate ER®, Concerta®, and Methylin ER®) 2000’s Introduction of methylphenidate or amphetamine Introduction of pemoline (Cylert®) and dextroamphetamine (Dexedrine®) More formulations of methylphenidate become available (Focalin®, Adderall XR®, Methadate CD®, Ritalin LA®, Methylin®, Focalin XR®, Daytrana®) as well as lisdexamfetamine (Vyvanse®) Release of non-stimulants atomoxetine (Strattera®), guanfacine (Intuniv®), and clonidine (Kapway®) PHARMACY CDC. ADHD: Data & Statistics. http: //www. cdc. gov/ncbddd/adhd/data. html

PHARMACY Pediatrics 2011; 128(5): SI 1 -SI 21.

PHARMACY N Engl J Med 2014; 370(9): 838 -846.

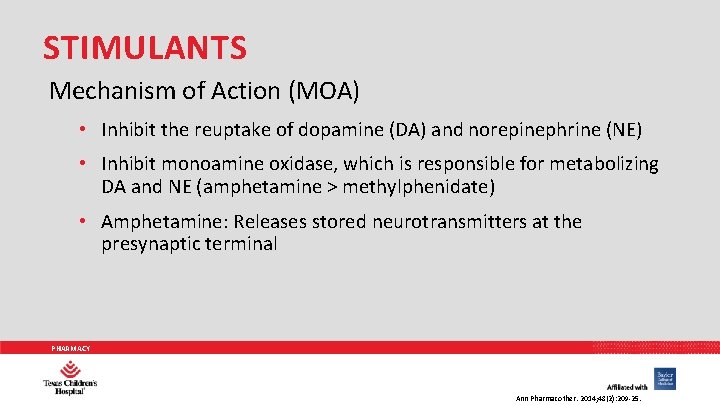
STIMULANTS Mechanism of Action (MOA) • Inhibit the reuptake of dopamine (DA) and norepinephrine (NE) • Inhibit monoamine oxidase, which is responsible for metabolizing DA and NE (amphetamine > methylphenidate) • Amphetamine: Releases stored neurotransmitters at the presynaptic terminal PHARMACY Ann Pharmacother. 2014; 48(2): 209 -25.

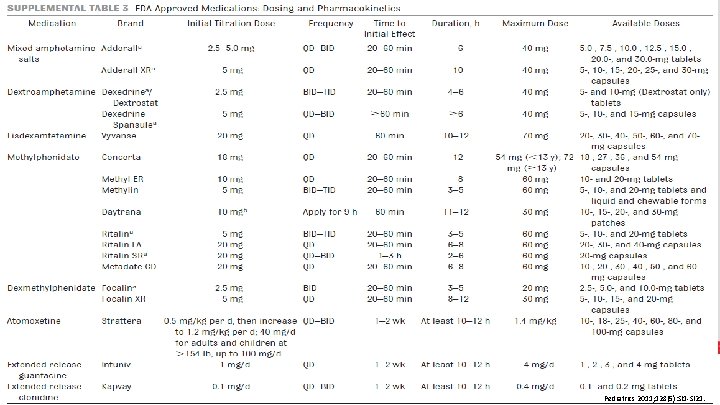
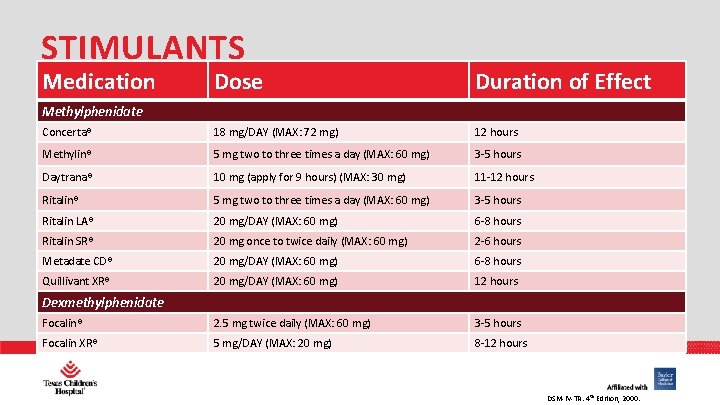
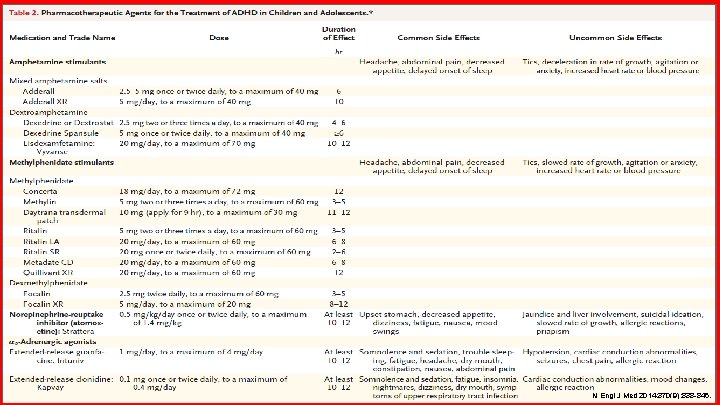
STIMULANTS Medication Dose Duration of Effect Concerta® 18 mg/DAY (MAX: 72 mg) 12 hours Methylin® 5 mg two to three times a day (MAX: 60 mg) 3 -5 hours Daytrana® 10 mg (apply for 9 hours) (MAX: 30 mg) 11 -12 hours Ritalin® 5 mg two to three times a day (MAX: 60 mg) 3 -5 hours Ritalin LA® 20 mg/DAY (MAX: 60 mg) 6 -8 hours Ritalin SR® 20 mg once to twice daily (MAX: 60 mg) 2 -6 hours Metadate CD® 20 mg/DAY (MAX: 60 mg) 6 -8 hours Quillivant XR® 20 mg/DAY (MAX: 60 mg) 12 hours Focalin® 2. 5 mg twice daily (MAX: 60 mg) 3 -5 hours Focalin XR® PHARMACY 5 mg/DAY (MAX: 20 mg) 8 -12 hours Methylphenidate Dexmethylphenidate DSM-IV-TR. 4 th Edition, 2000.

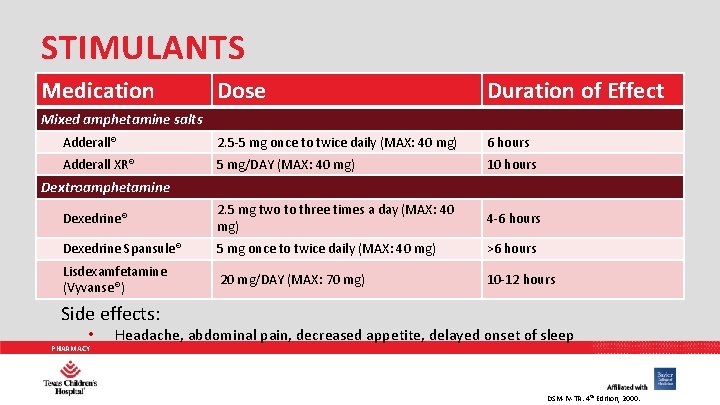
STIMULANTS Medication Dose Duration of Effect Adderall® 2. 5 -5 mg once to twice daily (MAX: 40 mg) 6 hours Adderall XR® 5 mg/DAY (MAX: 40 mg) 10 hours Dexedrine® 2. 5 mg two to three times a day (MAX: 40 mg) 4 -6 hours Dexedrine Spansule® 5 mg once to twice daily (MAX: 40 mg) >6 hours Lisdexamfetamine (Vyvanse®) 20 mg/DAY (MAX: 70 mg) 10 -12 hours Mixed amphetamine salts Dextroamphetamine Side effects: • PHARMACY Headache, abdominal pain, decreased appetite, delayed onset of sleep DSM-IV-TR. 4 th Edition, 2000.

METHYLPHENIDATE PHARMACY

IMMEDIATE RELEASE • Ritalin IR®, Methylin® • • • Initial dose: 2. 5 -5 mg/DAY (MAX: 60 mg/DAY) Peak: ~2 hours with a duration: 3 -6 hours Divide doses 2 -3 times/DAY (breakfast, lunch) PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

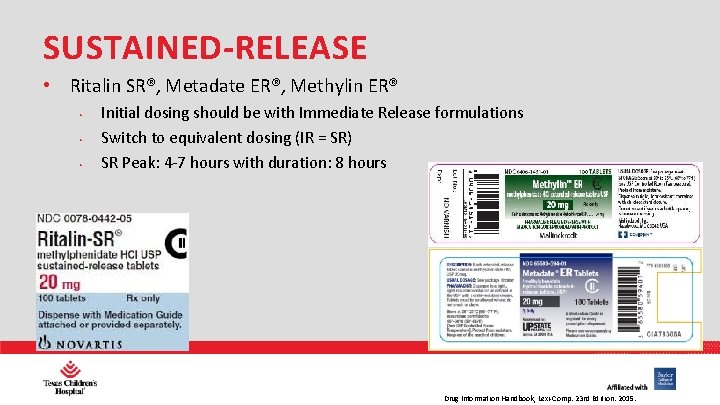
SUSTAINED-RELEASE • Ritalin SR®, Metadate ER®, Methylin ER® • • • Initial dosing should be with Immediate Release formulations Switch to equivalent dosing (IR = SR) SR Peak: 4 -7 hours with duration: 8 hours PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

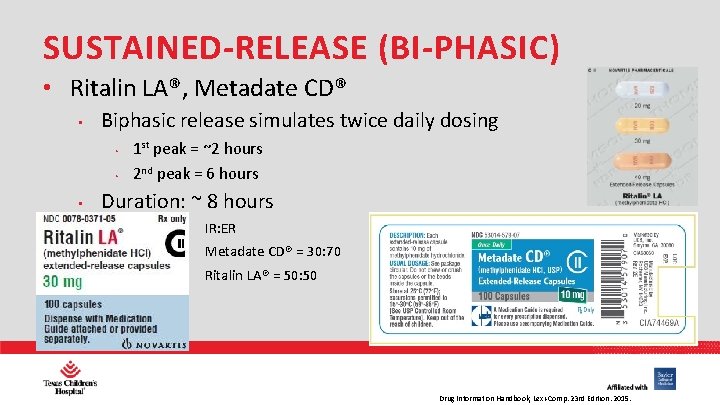
SUSTAINED-RELEASE (BI-PHASIC) • Ritalin LA®, Metadate CD® • Biphasic release simulates twice daily dosing • • • 1 st peak = ~2 hours 2 nd peak = 6 hours Duration: ~ 8 hours • IR: ER • Metadate CD® = 30: 70 • Ritalin LA® = 50: 50 PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

EXTENDED-RELEASE SUSPENSION • Quillivant XR® • Powder suspension for reconstitution • Shake well • Room temperature (4 months) • Final Concentration = 25 mg/5 m. L • Peak: ~4 hours • IR: ER (20: 80) PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

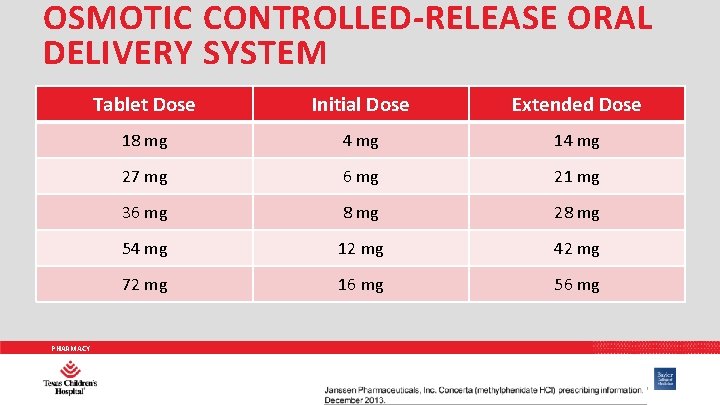
OSMOTIC CONTROLLED-RELEASE ORAL DELIVERY SYSTEM • Concerta® • • Dosing: 18 -72 mg/DAY Tri-phasic release stimulates three times daily dosing • 1 st peak = 1 -2 hours • Slow increase over next 3 -4 hours • Time to peak: 6 -8 hours • Slow decline • Duration: 12 hours • Adjust doses weekly PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

OSMOTIC CONTROLLED-RELEASE ORAL DELIVERY SYSTEM PHARMACY Tablet Dose Initial Dose Extended Dose 18 mg 4 mg 14 mg 27 mg 6 mg 21 mg 36 mg 8 mg 28 mg 54 mg 12 mg 42 mg 72 mg 16 mg 56 mg

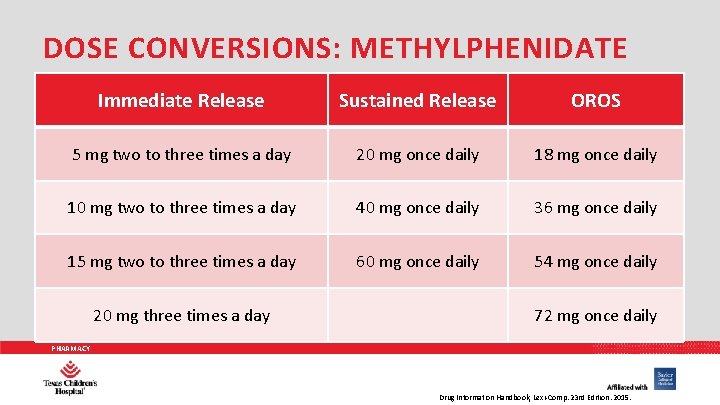
DOSE CONVERSIONS: METHYLPHENIDATE Immediate Release Sustained Release OROS 5 mg two to three times a day 20 mg once daily 18 mg once daily 10 mg two to three times a day 40 mg once daily 36 mg once daily 15 mg two to three times a day 60 mg once daily 54 mg once daily 20 mg three times a day 72 mg once daily PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

TRANSDERMAL PATCH • Daytrana® • Dosage Forms: 10, 15, 20, and 30 mg • Apply patch for 9 hours • Onset: ~2 hours • Peak: 7. 5 -10. 5 hours • Residual effects: 3 hours after removal PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

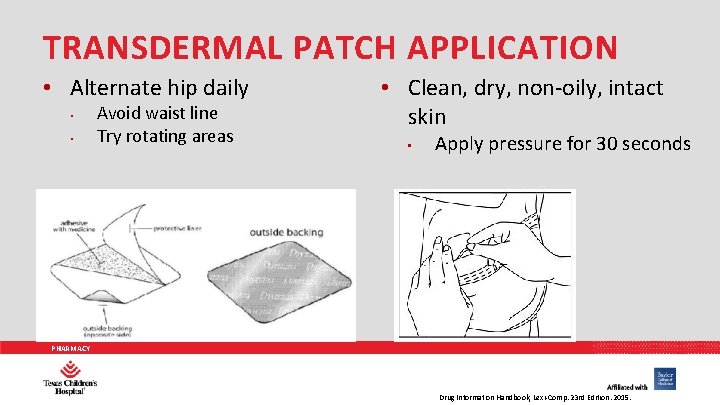
TRANSDERMAL PATCH APPLICATION • Alternate hip daily • • Avoid waist line Try rotating areas • Clean, dry, non-oily, intact skin • Apply pressure for 30 seconds PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

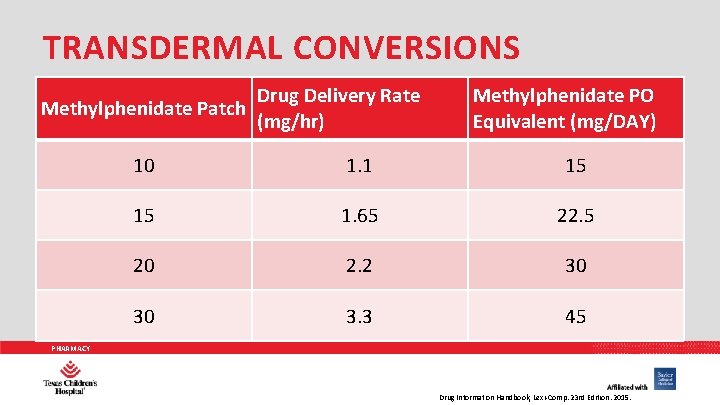
TRANSDERMAL CONVERSIONS Methylphenidate Patch Drug Delivery Rate (mg/hr) Methylphenidate PO Equivalent (mg/DAY) 10 1. 1 15 15 1. 65 22. 5 20 2. 2 30 30 3. 3 45 PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

DEXMETHYLPHENIDATE PHARMACY

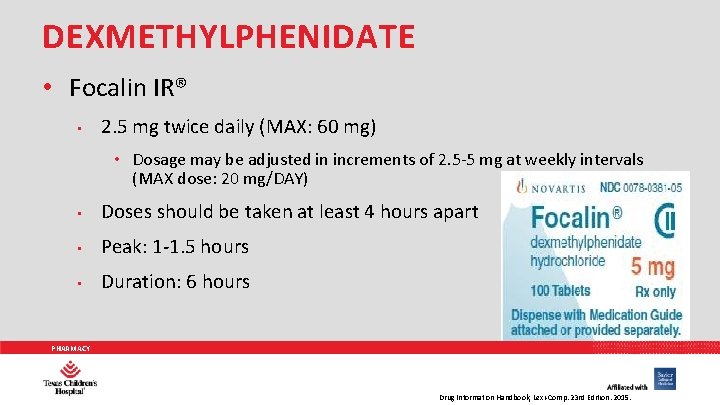
DEXMETHYLPHENIDATE • Focalin IR® • 2. 5 mg twice daily (MAX: 60 mg) • Dosage may be adjusted in increments of 2. 5 -5 mg at weekly intervals (MAX dose: 20 mg/DAY) • Doses should be taken at least 4 hours apart • Peak: 1 -1. 5 hours • Duration: 6 hours PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

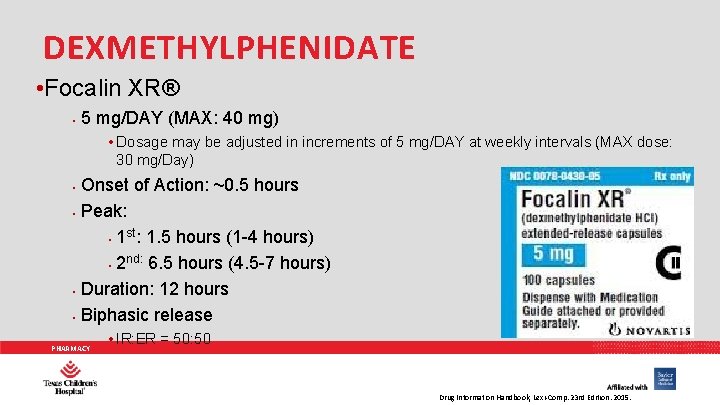
DEXMETHYLPHENIDATE • Focalin XR® • 5 mg/DAY (MAX: 40 mg) • Dosage may be adjusted in increments of 5 mg/DAY at weekly intervals (MAX dose: 30 mg/Day) • • Onset of Action: ~0. 5 hours Peak: st • 1 : 1. 5 hours (1 -4 hours) nd: 6. 5 hours (4. 5 -7 hours) • 2 Duration: 12 hours Biphasic release PHARMACY • IR: ER = 50: 50 Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

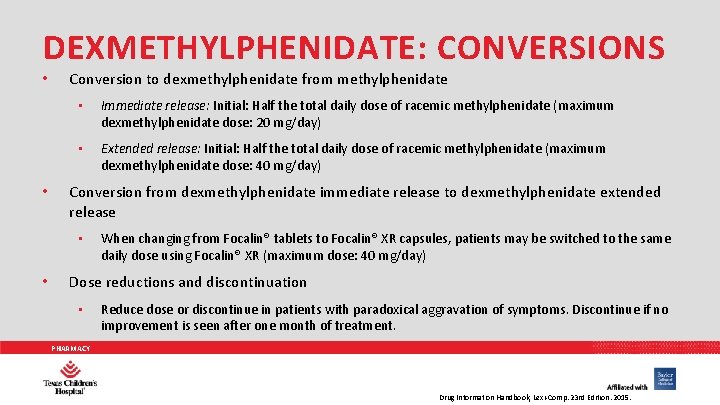
DEXMETHYLPHENIDATE: CONVERSIONS • • Conversion to dexmethylphenidate from methylphenidate • Immediate release: Initial: Half the total daily dose of racemic methylphenidate (maximum dexmethylphenidate dose: 20 mg/day) • Extended release: Initial: Half the total daily dose of racemic methylphenidate (maximum dexmethylphenidate dose: 40 mg/day) Conversion from dexmethylphenidate immediate release to dexmethylphenidate extended release • • When changing from Focalin® tablets to Focalin® XR capsules, patients may be switched to the same daily dose using Focalin® XR (maximum dose: 40 mg/day) Dose reductions and discontinuation • Reduce dose or discontinue in patients with paradoxical aggravation of symptoms. Discontinue if no improvement is seen after one month of treatment. PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

DEXTROAMPHETAMINE PHARMACY

DEXTROAMPHETAMINE • Dexedrine® • Dexedrine Spansules (SR)® • 5 mg/DAY (MAX: 20 mg) • 5 mg once daily (MAX: 15 mg) • Duration: 4 -6 hours • Duration: 6 -10 hours PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

LISDEXAMFETAMINE • Vyvanse® • Amphetamine pro-drug • Less potential for abuse • Dose: 20 -70 mg/DAY • Duration: 8 -12 hours • Capsules can be opened, dissolved in water and taken immediately PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

MIXED AMPHETAMINE SALTS PHARMACY

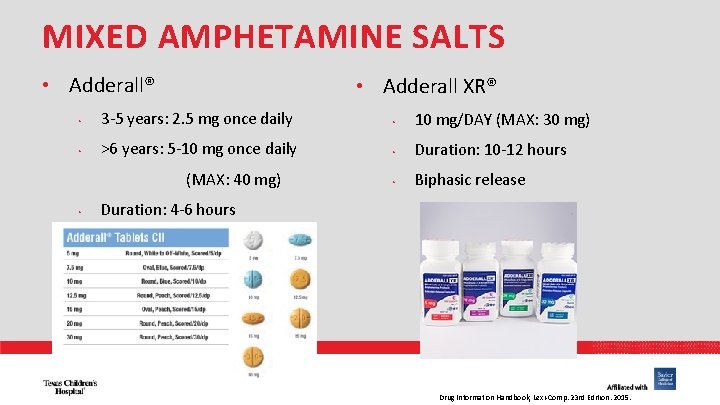
MIXED AMPHETAMINE SALTS • Adderall® • Adderall XR® • 3 -5 years: 2. 5 mg once daily • 10 mg/DAY (MAX: 30 mg) • >6 years: 5 -10 mg once daily • Duration: 10 -12 hours • Biphasic release (MAX: 40 mg) • Duration: 4 -6 hours PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

CONTRAINDICATIONS AND PRECAUTIONS FOR STIMULANTS • Anxiety • Seizure disorders • Motor tics • EEG abnormalities • MAO inhibitors • Cardiac abnormalities (or family history) • History of drug abuse • History of psychosis • Glaucoma PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

STIMULANTS: CLINICAL PEARLS • Drug holidays: Periodic discontinuation • • • Assess the patient's requirements Decrease tolerance Limit suppression of linear growth and weight Some patients may require 3 doses/DAY for treatment of ADHD Concerta® is OROS of methylphenidate Some products contain a mixture of immediate release and extended release beads • • Metadate CD® is designed to release 30% of the dose immediately and 70% over an extended period Ritalin LA® is designed to release 50% of the dose immediately and 50% delayed release over time • This is also enteric coated PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

STIMULANTS VS. NONSTIMULANTS Highly Effective Non-Scheduled • Rapid onset Schedule II • Side effects • Titration required PHARMACY Not as effective • Delayed onset • Side effects • Titration required

NONSTIMULANT MEDICATIONS FOR ADHD • Atomoxetine (Strattera®) • Clonidine ER tablets (Kapvay®) • Guanfacine ER tablets (Intuniv®) • Other non-FDA approved, off-label therapies: • Bupropion • Venlafaxine • TCAs PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

ATOMOXETINE • Norepinephrine reuptake inhibitor • Onset of effect: 2 -4 weeks • Side effects • • • N/V, anorexia, increased BP/HR, constipation, sedation, rare hepatotoxicity (bolded warning) Once daily WEIGHT-BASED dosing • 0. 5 -1. 4 mg/kg/DAY up to 70 kg (Target dose of 1. 2 -1. 4 mg/kg/DAY is recommended) • Start with lower dose (0. 5 mg/kg/DAY) for one week and titrate up, monitoring side effects Better tolerated dosing when given with dinner, may decrease GI upset and daytime sedation PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.
![FDA THE BOXED WARNING Pediatric U S Boxed Warning Use with caution in pediatric FDA: THE BOXED WARNING Pediatric: [U. S. Boxed Warning]: Use with caution in pediatric](https://slidetodoc.com/presentation_image/1c765dd31a695a7d6cf422610f2071a2/image-78.jpg)
FDA: THE BOXED WARNING Pediatric: [U. S. Boxed Warning]: Use with caution in pediatric patients; may be an increased risk of suicidal ideation. Closely monitor for clinical worsening, suicidality, or unusual changes in behavior; especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases. The family or caregiver should be instructed to closely observe the patient and communicate condition with healthcare provider. Growth should be monitored during treatment. Height and weight gain may be reduced during the first 9 to 12 months of treatment, but should recover by 3 years of therapy. PHARMACY

FDA: THE BOXED WARNING • Tell your child or teenager’s doctor if your child or teenager (or there is a family history of): • • Has bipolar illness (manic-depressive illness) Had suicide thoughts or actions before starting STRATTERA® • The chance for suicidal thoughts and actions may be higher: • • Early during STRATTERA® treatment During dose adjustments • Prevent suicidal thoughts and action in your child or teenager by: • • PHARMACY Paying close attention to your child or teenager’s moods, behaviors, thoughts, and feelings during STRATTERA® treatment Keeping all follow-up visits with your child or teenager’s doctor as scheduled

FDA: THE BOXED WARNING Watch for the following signs in your child or teenager during STRATTERA® treatment: • • • Anxiety Agitation Panic attacks Trouble sleeping Irritability Hostility PHARMACY • • • Aggressiveness Impulsivity Restlessness Mania Depression Suicidal thoughts

CLONIDINE / GUANFACINE • Alpha-adrenergic agonists • Extended release formulations • Approved as monotherapy or as adjunctive therapy in ADHD • Immediate release formulations • Not FDA approved, but see them used • Clinically useful for treating tics, hypertension, sleep problems, aggression, self-injurious behavior • More effective for impulsivity and hyperactivity than for inattentiveness symptoms PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

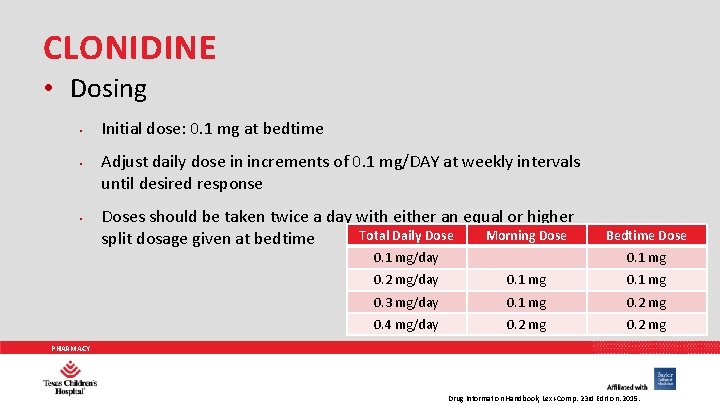
CLONIDINE • Dosing • • • Initial dose: 0. 1 mg at bedtime Adjust daily dose in increments of 0. 1 mg/DAY at weekly intervals until desired response Doses should be taken twice a day with either an equal or higher Total Daily Dose Morning Dose split dosage given at bedtime 0. 1 mg/day Bedtime Dose 0. 1 mg 0. 2 mg/day 0. 1 mg 0. 3 mg/day 0. 1 mg 0. 2 mg 0. 4 mg/day 0. 2 mg PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

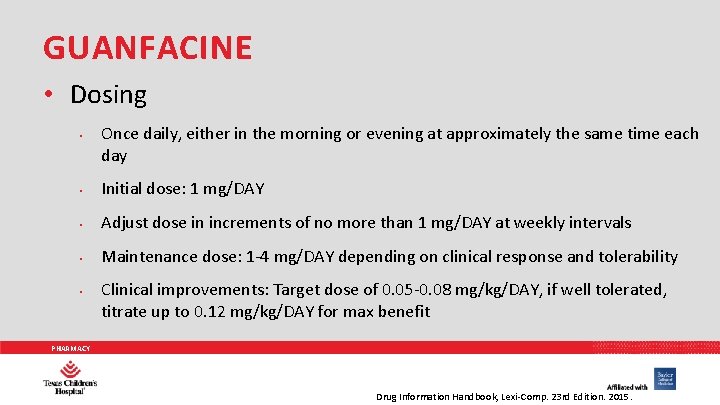
GUANFACINE • Dosing • Once daily, either in the morning or evening at approximately the same time each day • Initial dose: 1 mg/DAY • Adjust dose in increments of no more than 1 mg/DAY at weekly intervals • Maintenance dose: 1 -4 mg/DAY depending on clinical response and tolerability • Clinical improvements: Target dose of 0. 05 -0. 08 mg/kg/DAY, if well tolerated, titrate up to 0. 12 mg/kg/DAY for max benefit PHARMACY Drug Information Handbook, Lexi-Comp. 23 rd Edition. 2015.

PHARMACY Ann Pharmacother. 2014; 48(2): 209 -25.

PHARMACY N Engl J Med 2014; 370(9): 838 -846.

SUMMARY What should you ask yourself when treating ADHD? • Are the medications working? • Are the medications at appropriate doses? • Are the medications prescribed correctly? • Is the patient undergoing psychotherapy? • Is the patient being compliant? • Is the patient taking the medications at the correct time? PHARMACY

PHARMACY

COMMENTS/QUESTIONS? PHARMACY