SCH 4 U Grade 12 Chemistry Key Unit









![Rate Equation rate = [B] for substance “B” t rate = - [A] for Rate Equation rate = [B] for substance “B” t rate = - [A] for](https://slidetodoc.com/presentation_image_h2/583e954dbf6b4e9013509a7c45e63b9b/image-17.jpg)



- Slides: 28

SCH 4 U Grade 12 Chemistry

Key Unit Learning Goals • I will recognize that rates of chemical reactions vary • I will be able to identify factors that affect the rate of a reaction • I will describe how rate of disappearance of a reactant and appearance of a product are related • I will be able to explain the concept of a reaction mechanism • I will use collision theory to explain why reaction rates increase or decrease

Kaboom! Or……

…. Ka-Save my<3 ? ? ! Vasodilator (actually gross)

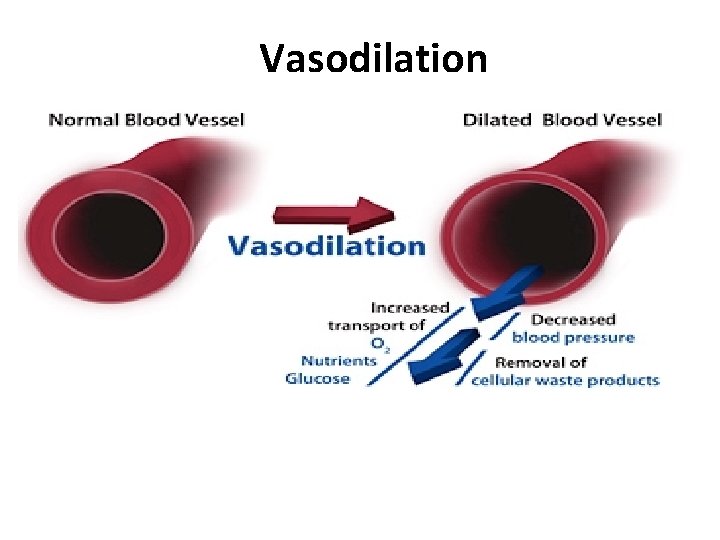
Vasodilation

PRE WORKOUTS! • Pre-workouts are vasodilators as well • Contain the amino acid Arginine, and the pseudo-amino acid Citrulline.

Citrulline • Watermelons are good source of citrulline

Red Beets • Red beets are amazing vasodilators as well • Used by endurance athletes

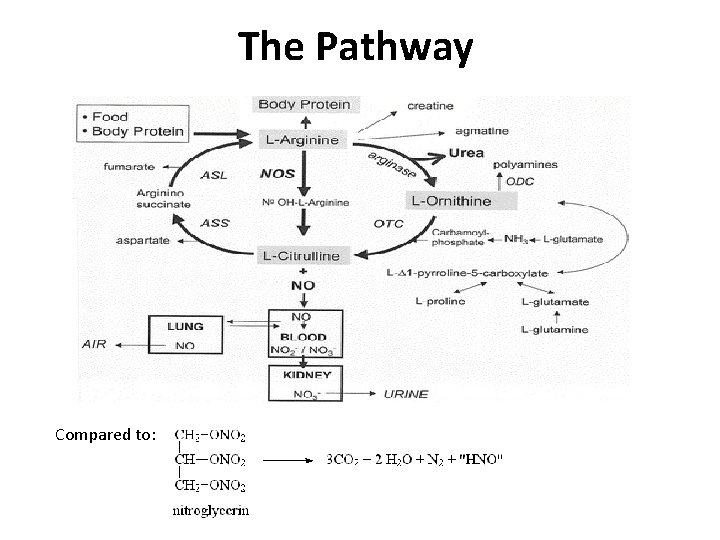
The Pathway Compared to:

Learning Goals • I will understand what a reaction rate is • I will be able to calculate the average reaction rate of a chemical reaction • I will be able to determine the average reaction rate using graphical data • I will know how to find the instantaneous rate of reaction • I will understand the stoichiometric rate relationships for rate reactions

Reaction Rates • The reaction rate (rate of reaction) or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place.

Sloooooow • Example: the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can take many years

Fst! • Combustion of cellulose (wood) in a fire is a reaction that takes place in fractions of a second

Reaction Rates • Reaction rates are usually expressed as the concentration of reactant consumed or the concentration of product formed per unit time. • The units are thus moles per liter per unit time, written as M/s

But How? • To measure reaction rates, chemists initiate the reaction, measure the concentration of the reactant or product at different times as the reaction progresses, perhaps plot the concentration as a function of time on a graph, and then calculate the change in the concentration per unit time.

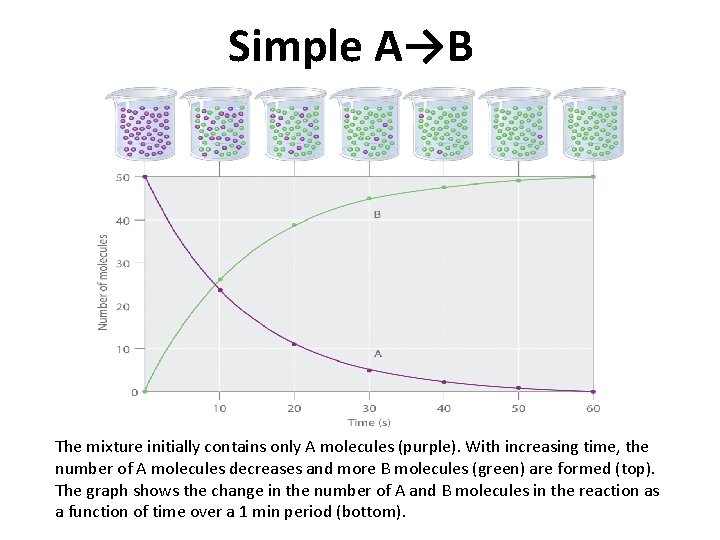
Simple A→B The mixture initially contains only A molecules (purple). With increasing time, the number of A molecules decreases and more B molecules (green) are formed (top). The graph shows the change in the number of A and B molecules in the reaction as a function of time over a 1 min period (bottom).
![Rate Equation rate B for substance B t rate A for Rate Equation rate = [B] for substance “B” t rate = - [A] for](https://slidetodoc.com/presentation_image_h2/583e954dbf6b4e9013509a7c45e63b9b/image-17.jpg)
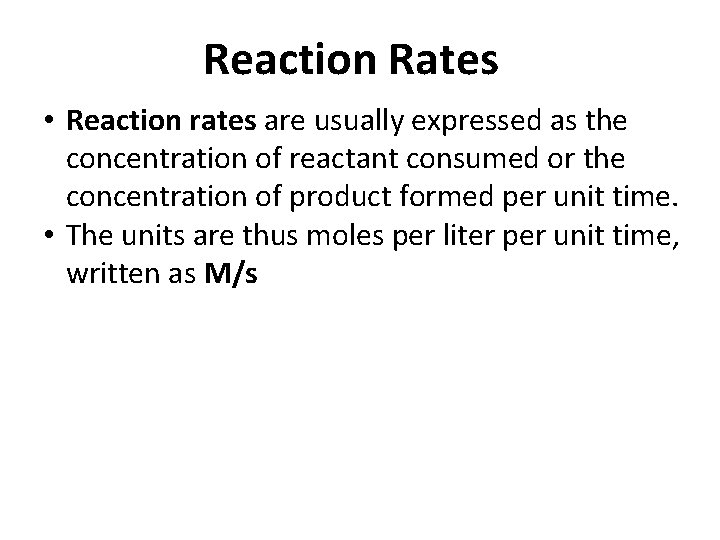
Rate Equation rate = [B] for substance “B” t rate = - [A] for substance “A” t • square brackets indicate molar concentrations • capital Greek delta (Δ) means “change in” • chemists follow the convention of expressing all reaction rates as positive numbers, however, a negative sign is inserted in front of Δ[A]/Δt to convert that expression to a positive number

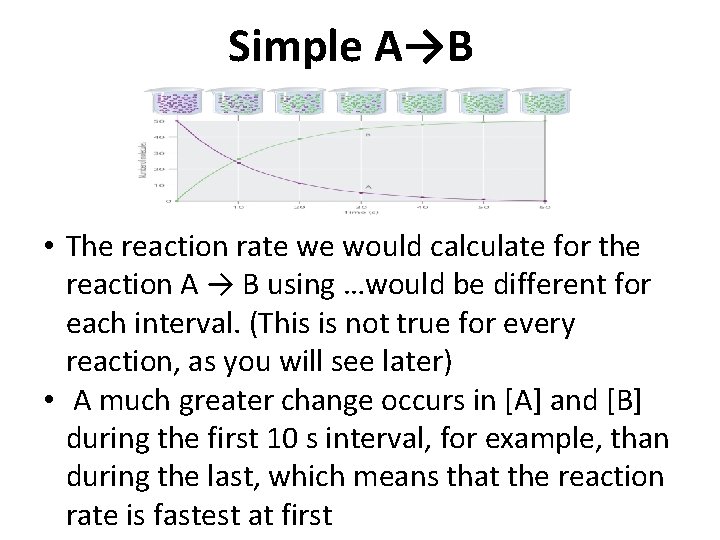
Simple A→B • The reaction rate we would calculate for the reaction A → B using …would be different for each interval. (This is not true for every reaction, as you will see later) • A much greater change occurs in [A] and [B] during the first 10 s interval, for example, than during the last, which means that the reaction rate is fastest at first

Determining Reaction Rate of Aspirin • We can use the previous equation to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used drug in the world. • More than 25, 000 kg are produced annually worldwide. Aspirin (acetylsalicylic acid) reacts with water (such as water in body fluids) to give salicylic acid and acetic acid.

Determining Reaction Rate of Aspirin

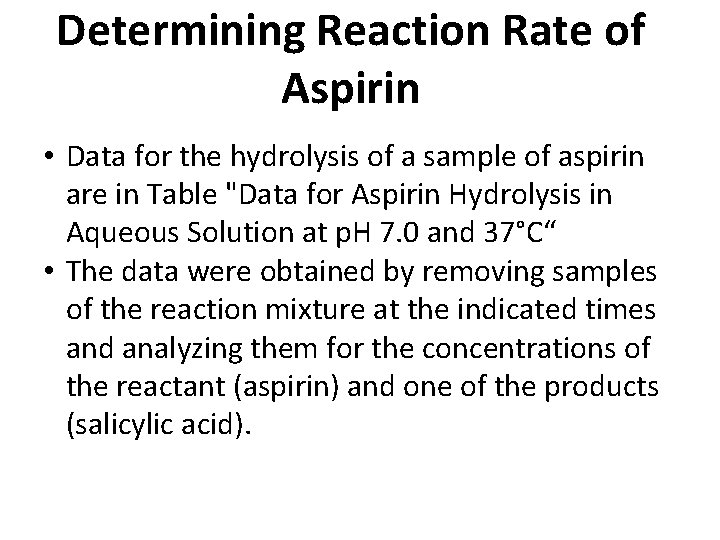
Determining Reaction Rate of Aspirin • Data for the hydrolysis of a sample of aspirin are in Table "Data for Aspirin Hydrolysis in Aqueous Solution at p. H 7. 0 and 37°C“ • The data were obtained by removing samples of the reaction mixture at the indicated times and analyzing them for the concentrations of the reactant (aspirin) and one of the products (salicylic acid).

Determining Reaction Rate of Aspirin Data for Aspirin Hydrolysis in Aqueous Solution at p. H 7. 0 and 37°C Time (h) 0 2. 0 5. 0 10 20 30 40 50 100 200 300 [Aspirin] (M) [Salicylic Acid] (M) 5. 55 × 10− 3 0 5. 51 × 10− 3 0. 040 × 10− 3 5. 45 × 10− 3 0. 10 × 10− 3 5. 35 × 10− 3 0. 20 × 10− 3 5. 15 × 10− 3 0. 40 × 10− 3 4. 96 × 10− 3 0. 59 × 10− 3 4. 78 × 10− 3 0. 77 × 10− 3 4. 61 × 10− 3 0. 94 × 10− 3 3. 83 × 10− 3 1. 72 × 10− 3 2. 64 × 10− 3 2. 91 × 10− 3 1. 82 × 10− 3 3. 73 × 10− 3 Slooooooooow!

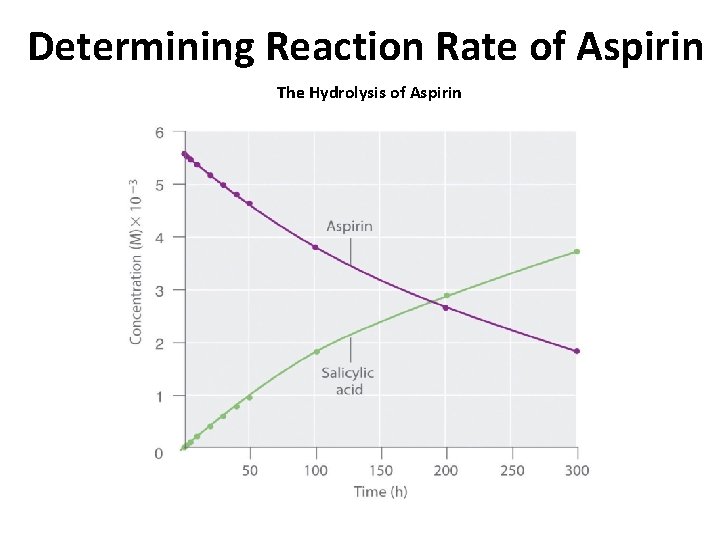
Determining Reaction Rate of Aspirin The Hydrolysis of Aspirin

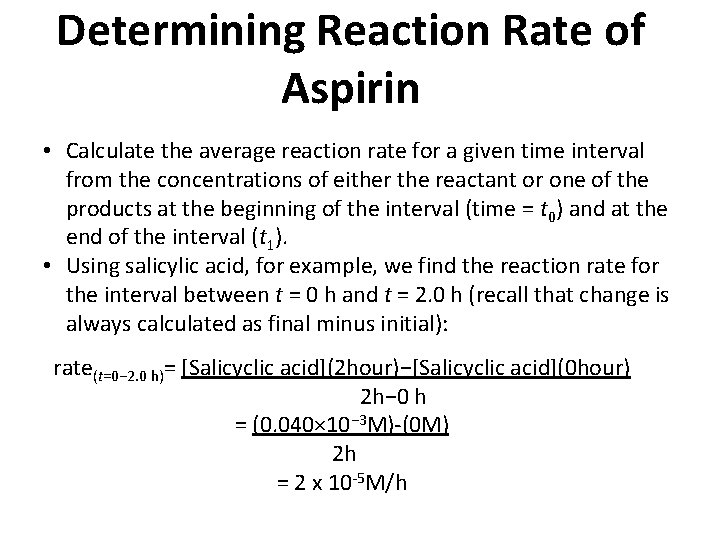
Determining Reaction Rate of Aspirin • Calculate the average reaction rate for a given time interval from the concentrations of either the reactant or one of the products at the beginning of the interval (time = t 0) and at the end of the interval (t 1). • Using salicylic acid, for example, we find the reaction rate for the interval between t = 0 h and t = 2. 0 h (recall that change is always calculated as final minus initial): rate(t=0− 2. 0 h)= [Salicyclic acid](2 hour)−[Salicyclic acid](0 hour) 2 h− 0 h = (0. 040× 10− 3 M)-(0 M) 2 h = 2 x 10 -5 M/h

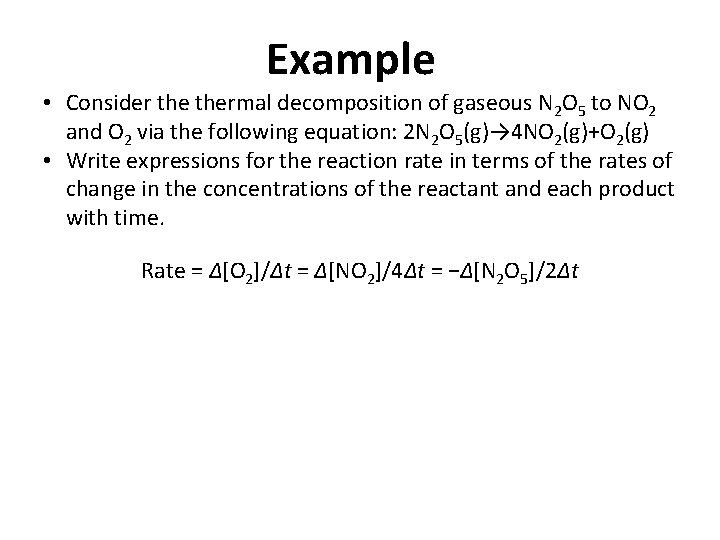
Example • Consider thermal decomposition of gaseous N 2 O 5 to NO 2 and O 2 via the following equation: 2 N 2 O 5(g)→ 4 NO 2(g)+O 2(g) • Write expressions for the reaction rate in terms of the rates of change in the concentrations of the reactant and each product with time. Rate = Δ[O 2]/Δt = Δ[NO 2]/4Δt = −Δ[N 2 O 5]/2Δt

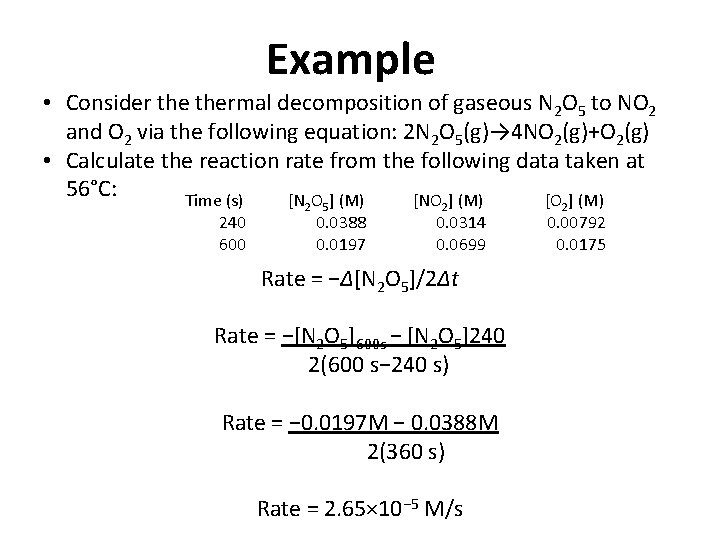
Example • Consider thermal decomposition of gaseous N 2 O 5 to NO 2 and O 2 via the following equation: 2 N 2 O 5(g)→ 4 NO 2(g)+O 2(g) • Calculate the reaction rate from the following data taken at 56°C: Time (s) [N O ] (M) [NO ] (M) [O ] (M) 240 600 2 5 0. 0388 0. 0197 2 0. 0314 0. 0699 Rate = −Δ[N 2 O 5]/2Δt Rate = −[N 2 O 5]600 s − [N 2 O 5]240 2(600 s− 240 s) Rate = − 0. 0197 M − 0. 0388 M 2(360 s) Rate = 2. 65× 10− 5 M/s 2 0. 00792 0. 0175

Instantaneous Rate • So far, we have determined average reaction rates over particular intervals of time. • We can also determine the instantaneous rate of a reaction, which is the reaction rate at any given point in time. • The instantaneous rate of a reaction at a given time corresponds to the slope of a line tangent to the concentration-versus-time curve at that point

• Look over 6. 1 • Look at Pg. 360: 1, 2 • Pg. 361: 1 -4