SCAI Publications Manual of Standard Operating Procedures Approved

SCAI Publications Manual of Standard Operating Procedures Approved by the SCAI Board of Trustees in 2019

Published in CCI • Ensures transparency of policies and processes • Available for ongoing reference by stakeholders (writing groups, committee members, leadership, collaborating organizations, SCAI membership, document audiences) Szerlip, M, Feldman, DN, Aronow, HD, et al. SCAI publications committee manual of standard operating procedures. Catheter Cardiovasc Interv. 2020; 1– 11. https: //doi. org/10. 1002/ccd. 28754
Disclosure & management of COI Multistakeholder engagement High. Impact Guidelines Innovative methodology Strategic topic prioritization

Scope & Contents of the Manual 1. Publications Committee 2. Terms & definitions 3. Topic identification & prioritization 4. Collaboration with other organizations 5. COI disclosure & management 6. Writing group formation 7. Methodology for document development 8. Peer review & public comment 9. Endorsement 10. Evaluation & maintenance

Publications Committee Mission Statement: To promote optimal patient care through educational, policy, and clinical/scientific documents that reflect the current state-of-the-science in interventional cardiology. Charge: Direct the initiation and development of SCAI position statements and documents to provide evidence-based guidance for clinical practice.

Types of SCAI Guidance Documents Clinical practice guideline: Recommendations that are informed by a systematic review of evidence and a structured, explicit assessment of the benefits and harms of an intervention Expert consensus statement: Recommendations that provide guidance on a controversial clinical topic developed through a systematic process for achieving consensus, often with limited available data Position statement: An official society position or opinion formulated by an expert writing group
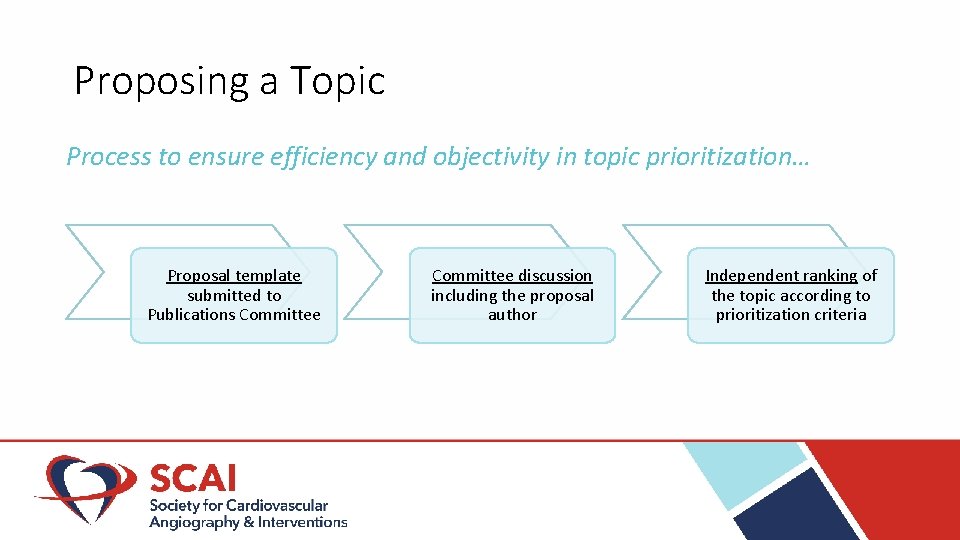
Proposing a Topic Process to ensure efficiency and objectivity in topic prioritization… Proposal template submitted to Publications Committee discussion including the proposal author Independent ranking of the topic according to prioritization criteria

Criteria for Topic Prioritization Disease burden: incidence/prevalence, impact on health care system, frequency of risk factors, avoidable morbidity/mortality, impact on individual patient quality of life Variation in clinical practice: uncertainty or controversy resulting in disparities in patient care Practice evolution: rapidly changing data or technology may impact the decision about if/when to initiate a document Availability of evidence: although it is important to estimate whether data are available to inform recommendations, it may also be desirable to undertake document development with the purpose of guiding research priorities and helping clinicians make the best use of limited evidence Redundancy/overlap: availability/currency of guidance from SCAI or another organization Feasibility: capacity of the society to undertake development and dissemination of the recommendations

Collaboration with Other Organizations • Identified before development begins based on the expected audience of the document (proposal stakeholder analysis) • Approved through the Publications Committee • Follow a prespecified model to delineate the roles and expectations for each organization
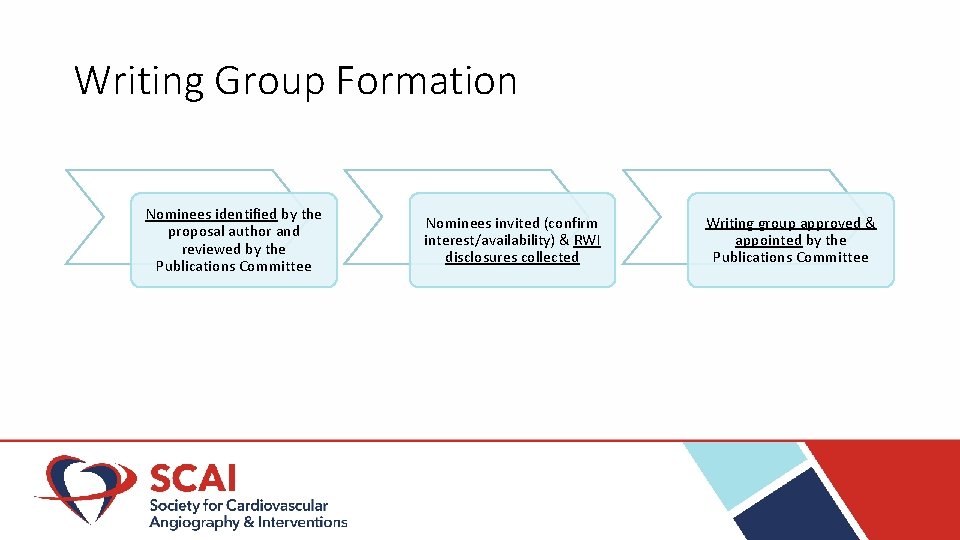
Writing Group Formation Nominees identified by the proposal author and reviewed by the Publications Committee Nominees invited (confirm interest/availability) & RWI disclosures collected Writing group approved & appointed by the Publications Committee

Writing Group Member Responsibilities • • Attend conference calls and/or in-person meetings Participate in evidence review & appraisal Vote on decisions and recommendations Write portions of the document manuscript Review drafts of material prepared by other authors Approve the final document Respond to action items on time according to deadlines agreed upon by the group

Additional Responsibilities of the Chair & Vice-Chair • Facilitate group discussions • Build consensus around decisions • Assist writing group members to comply with SCAI confidentiality, disclosure & recusal policies • Respond to feedback collected during the review period • Determine the order of authors & prepare the final manuscript for organizational approval & publication

Conflict of interest in the media…

Collection & Management of Disclosure Information Before development begins… • Nominees disclose intellectual & financial relationships from the prior 12 months before they are appointed to a writing group • Disclosure info is vetted by the Publications Committee ü Writing group must be balanced with a majority (50% + 1) of members who do not have significant conflicts* ü The document Chair cannot have significant conflicts* *Criteria for significant conflicts are defined in the SCAI Policy for Management of Relationships with Industry (RWI) posted on the society website

Collection & Management of Disclosure Information During development… • Writing group reviews all disclosure information prior to each meeting • Group members are required to update their disclosures with any changes that occur during development; initiating new relationships is strongly discouraged • Group members with relationships that are relevant to the document content are recused from participating in related discussions and votes

Collection & Management of Disclosure Information Upon publication… • The process for collection and management of disclosures is reported in the document manuscript • Disclosure information, including any updates that occurred during the development process, is published as an appendix to the final document

Confidentiality All aspects of the document development process are confidential until publication of the resulting manuscript. This includes the content of writing group discussions, materials circulated among writing group members, and content of the document. Writing group members should refrain from commenting about the development effort in public, including to members of the media and industry. If in doubt, assume it’s confidential.

Methodology: from evidence to recommendations • Each document follows a predefined process for collecting and synthesizing evidence, conducting writing group deliberations, and reporting on group decisions – described in the SOP • Recommendations are presented in a standard format across all SCAI documents • Each manuscript reports the following elements: masthead, abstract, introduction/background, methods, analysis, conclusion

Peer Review & Public Comment • Capture stakeholder perspectives that are not represented on the writing group (including gathering additional perspectives from SCAI members) • Documents that are expected to have broad, practice-changing effects may undergo public comment: • Anticipate controversy and respond with an appropriate implementation strategy • Enhance the transparency of the document development process • First step in raising awareness and disseminating the recommendations

Endorsement • Required for any clinical guidance document to carry the imprimatur of SCAI, including SCAI-sponsored documents and documents that are sponsored by other organizations. • Two-step process: 1. Publications Committee approval 2. Executive Committee approval • Any document developed according to the processes and policies described in the SOP has met the criteria for endorsement by SCAI.

For more information… • Refer to the SOP library on the SCAI website: üManual of Standard Operating Procedures (SOP) üTopic Proposal Template üDisclosure Form üPolicy for Management of Relationships with Industry (RWI) http: //www. scai. org/Guidelines/ • Contact the Publications Committee Chair, Co-Chair, and staff liaison
- Slides: 21