SCAI Guidelines on Device Selection in AortoIliac Arterial


- Slides: 12

SCAI Guidelines on Device Selection in Aorto‐Iliac Arterial Interventions Slides Developed By: Dmitriy N. Feldman, MD, FSCAI, Document Chair Director, Endovascular Service Director, Interventional Observation/Telemetry Unit Associate Professor of Medicine Interventional Cardiac and Endovascular Laboratory Weill Cornell Medical College, New York Presbyterian Hospital

SCAI Guidelines on Device Selection in Aorto‐Iliac Arterial Interventions Dmitriy N. Feldman MD, FSCAI (Chair); Ehrin J. Armstrong MD, FSCAI; Herbert D. Aronow MD, MPH, FSCAI; Subhash Banerjee MD, FSCAI; Larry J. Díaz‐Sandoval MD, FSCAI; Michael R. Jaff DO, FSCAI; Sasanka Jayasuriya MD, FSCAI; Safi U. Khan MD, MS; Andrew J. Klein MD, FSCAI; Sahil A. Parikh MD, FSCAI; Kenneth Rosenfield MD, MSCAI; Mehdi H. Shishehbor DO, MPH, Ph. D, FSCAI; Rajesh V. Swaminathan MD, FSCAI; Christopher J. White MD, MSCAI (Vice‐Chair) Feldman, DN, Armstrong, EJ, Aronow, HD, et al. SCAI guidelines on device selection in Aorto‐Iliac arterial interventions. Catheter Cardiovasc Interv. 2020; 1– 15. https: //doi. org/10. 1002/ccd. 28947

Scope & Aims • Provide a comprehensive review of comparative effectiveness data in aorto-iliac arterial interventions • Focus on safety and efficacy of groups of devices • Consider cost as a secondary domain after examining efficacy and safety data • Provide clinicians with transparent guidance (class of recommendation and level of evidence) for device selection, when these devices are intended as definitive therapy.

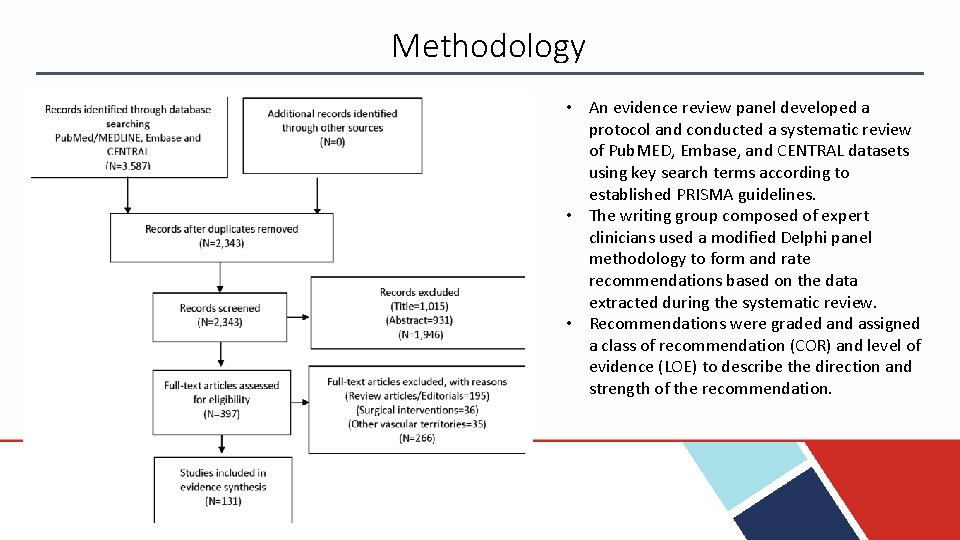
Methodology • An evidence review panel developed a protocol and conducted a systematic review of Pub. MED, Embase, and CENTRAL datasets using key search terms according to established PRISMA guidelines. • The writing group composed of expert clinicians used a modified Delphi panel methodology to form and rate recommendations based on the data extracted during the systematic review. • Recommendations were graded and assigned a class of recommendation (COR) and level of evidence (LOE) to describe the direction and strength of the recommendation.

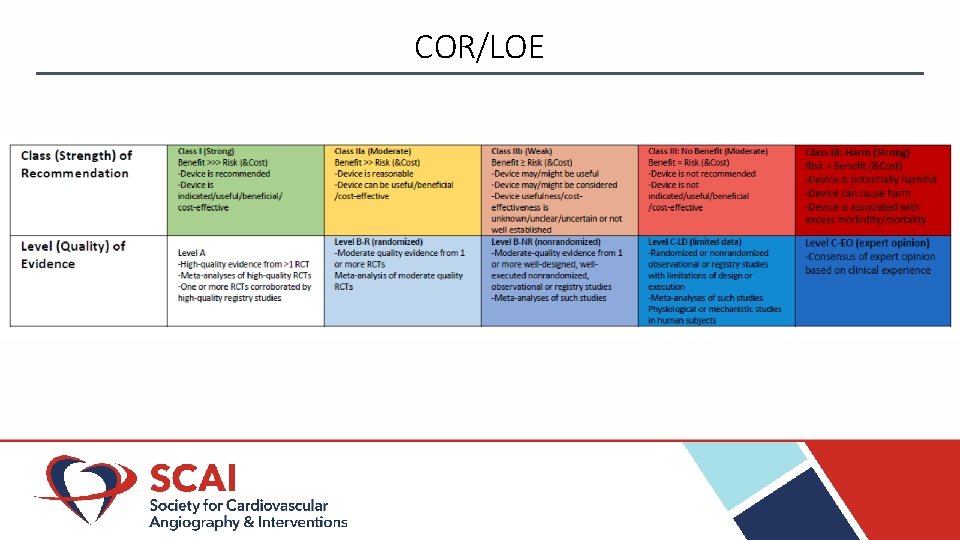
COR/LOE

Indications for Aorto‐Iliac Interventions AORTO-ILIAC DISEASE WITH SYMPTOMS • • • Relieve claudication Wound healing in CLI Improve functional status and Quality of Life (QOL) AORTO-ILIAC DISEASE WITHOUT SYMPTOMS • Situations where large-bore arterial access is required for hemodynamic support devices (e. g. , IABP or other catheter-based ventricular assist devices), for structural/valvular (e. g. , TAVR), and vascular (e. g. , endovascular aortic aneurysm repair) procedures Klein A, Jaff M, Gray B et al. Catheter Cardiovasc Interv. 2017; 90: E 90–E 110.

Aorto‐Iliac Revascularization Device Options • • • Uncoated balloon PTA Specialty balloon PTA DCB BMS (balloon expandable) BMS (self-expanding) Covered stent (balloon expandable) Covered stent (self-expanding) DES Atherectomy

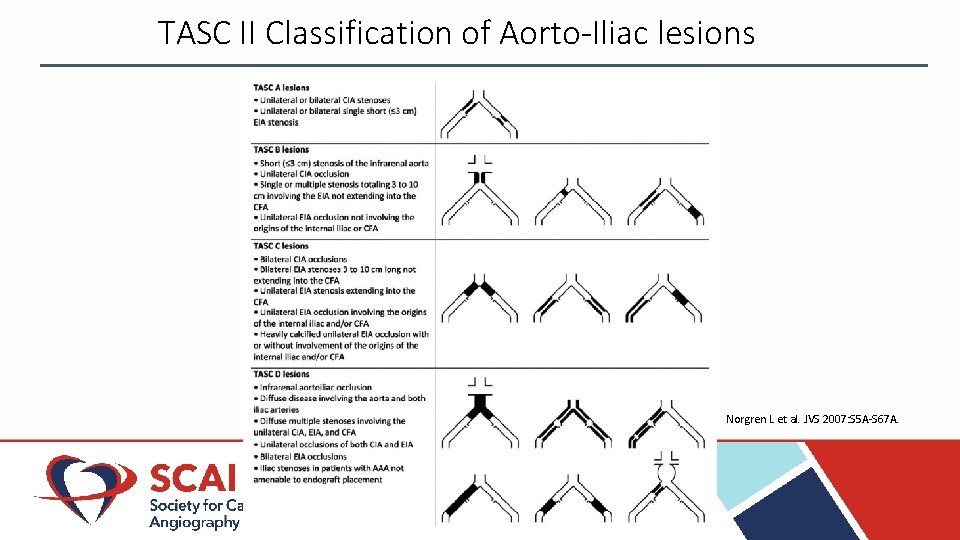
TASC II Classification of Aorto‐Iliac lesions Norgren L et al. JVS 2007: S 5 A-S 67 A.

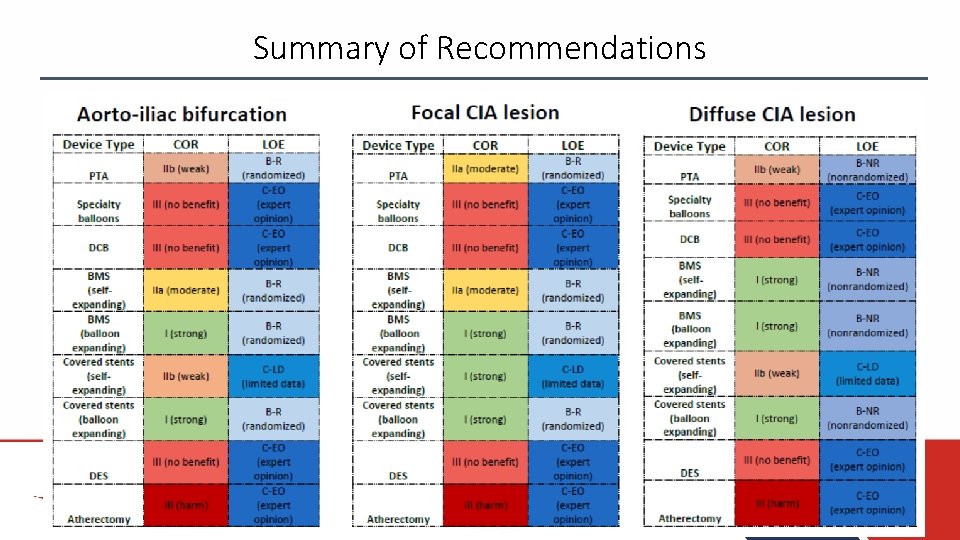
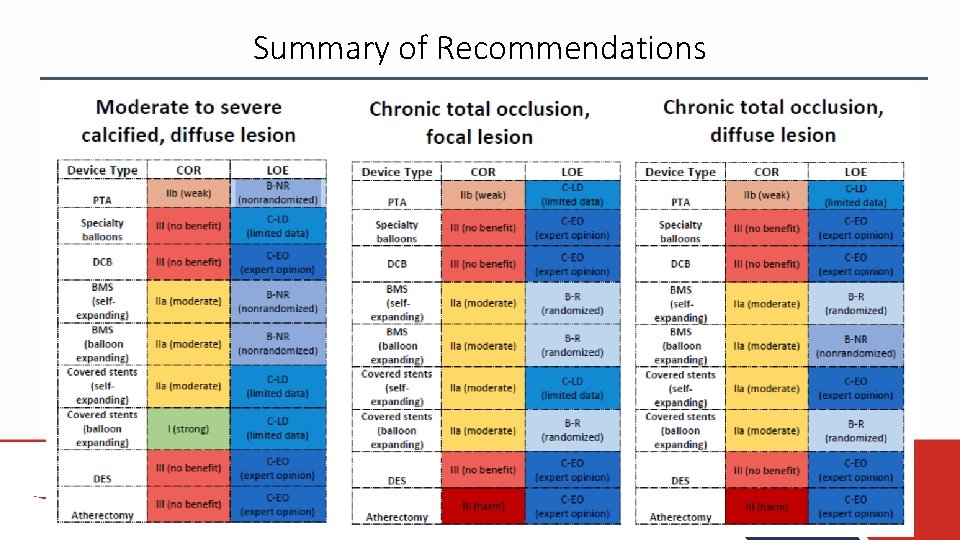
Summary of Recommendations

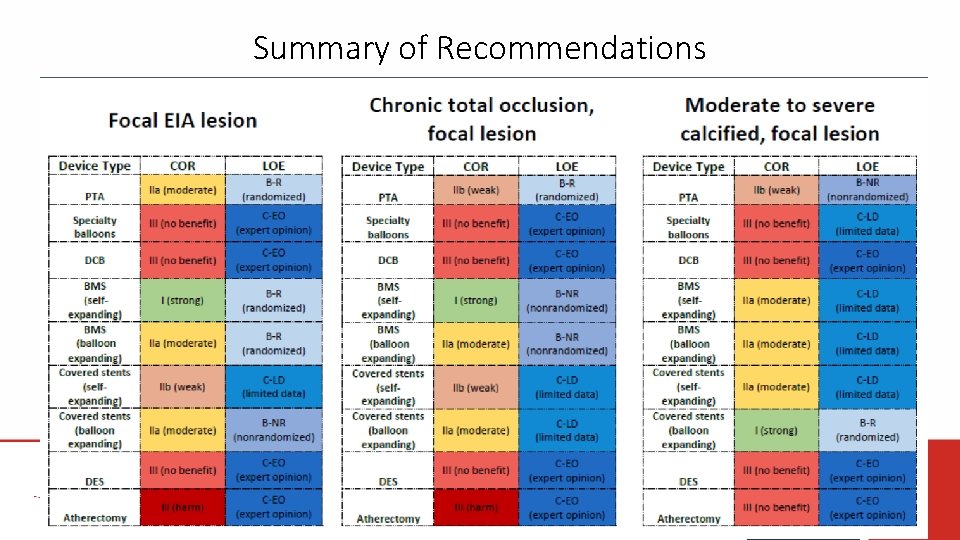
Summary of Recommendations

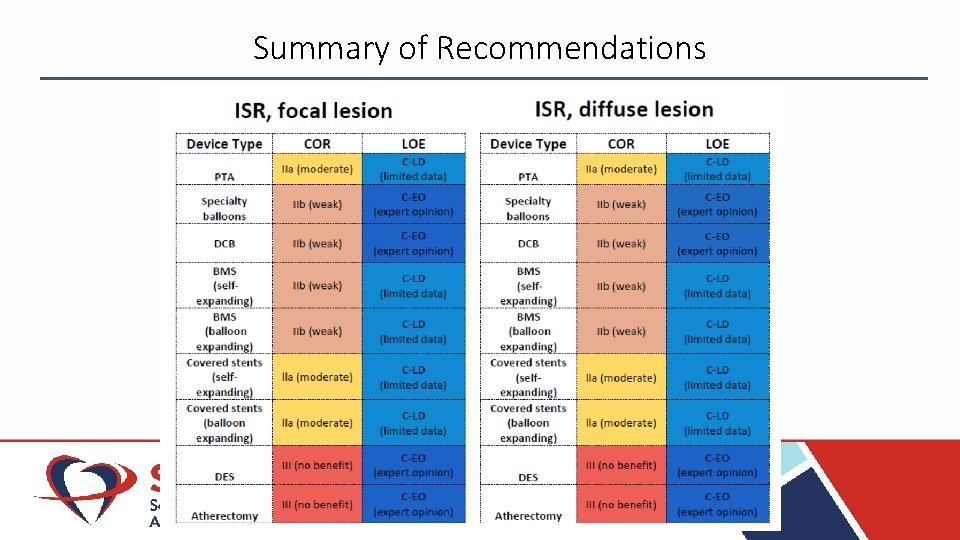
Summary of Recommendations

Summary of Recommendations