SBP Prophylaxis Lenny Chen PGY2 5132015 Outline Patient

SBP Prophylaxis Lenny Chen PGY-2 5/13/2015

Outline Patient history – Case presentation Clinical questions Introduction/Background Information Journal article Critical Appraisal Limitations Conclusion

My Patient 54 year old Caucasian Lady with a PMH of uncontrolled T 2 DM, medical non-compliance, retinopathy, Hep C cirrhosis, hypertension, bipolar disorder, and hypothyroidism. Pt had recent paracentesis proven SBP a few months ago require 1 week of ciprofloxacin treatment.

My Patient comes for Inpatient follow up. She has nausea to ciprofloxacin would not take it for long term. Pt still has a non healing diabetic foot ulcer with multiple episodes of cellulitis.

Clinical Questions PICO question P: Known cirrhotic patient with Hx of SBP I: another Antibiotic C: Norfloxacin O: difference in secondary SBP prophylaxis Search Term: Antibiotics SBP prophylaxis

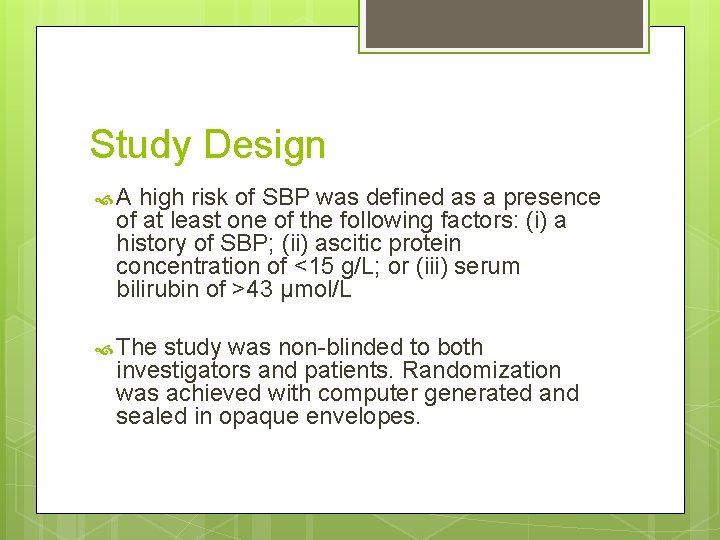
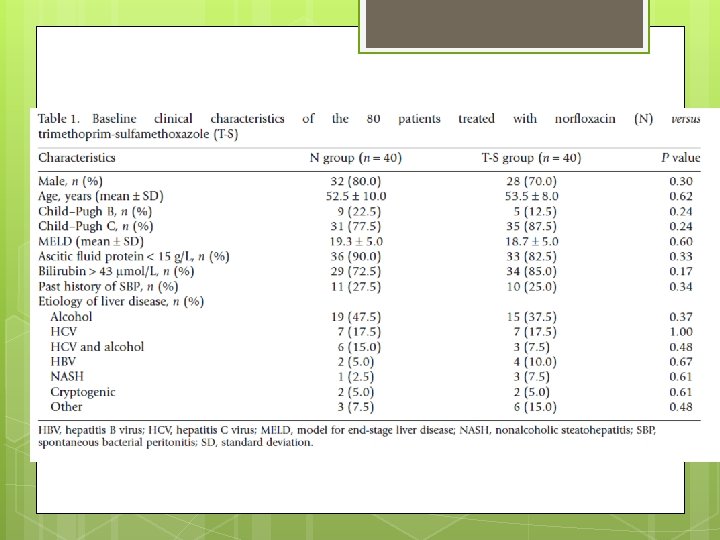
Study Design A high risk of SBP was defined as a presence of at least one of the following factors: (i) a history of SBP; (ii) ascitic protein concentration of <15 g/L; or (iii) serum bilirubin of >43 μmol/L The study was non-blinded to both investigators and patients. Randomization was achieved with computer generated and sealed in opaque envelopes.
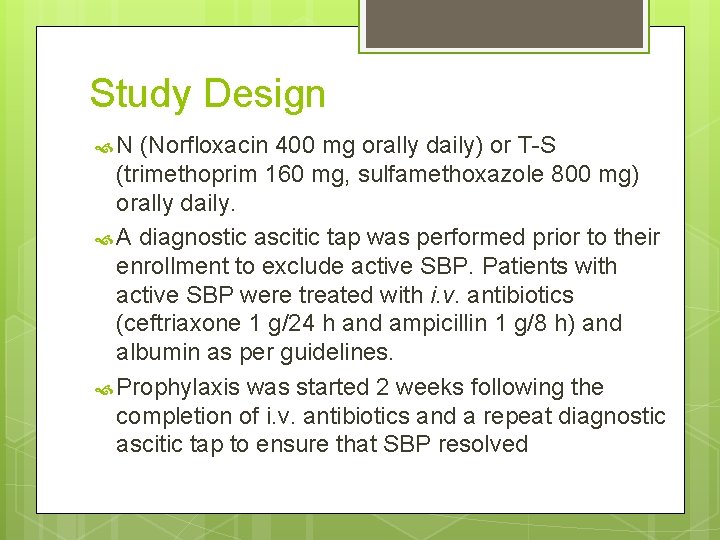
Study Design N (Norfloxacin 400 mg orally daily) or T-S (trimethoprim 160 mg, sulfamethoxazole 800 mg) orally daily. A diagnostic ascitic tap was performed prior to their enrollment to exclude active SBP. Patients with active SBP were treated with i. v. antibiotics (ceftriaxone 1 g/24 h and ampicillin 1 g/8 h) and albumin as per guidelines. Prophylaxis was started 2 weeks following the completion of i. v. antibiotics and a repeat diagnostic ascitic tap to ensure that SBP resolved

Study Design Followed up at 3 -month intervals or during any hospitalization. A repeat abdominal paracentesis was performed as clinically indicated SBP was defined as an ascitic fluid neutrophil count of ≥ 250

Study Exclusion Criteria (i) allergies to sulfurcontaining drugs or quinolones; (ii) documented failure of either drug in the past (iii) antibiotic therapy in the 2 weeks prior to the inclusion; (iv) severe renal impairment, defined as a creatinine clearance of less than 15 m. L/min GFR (v) the presence of hepatocellular carcinoma or other conditions with an expected survival of less than 3 months;
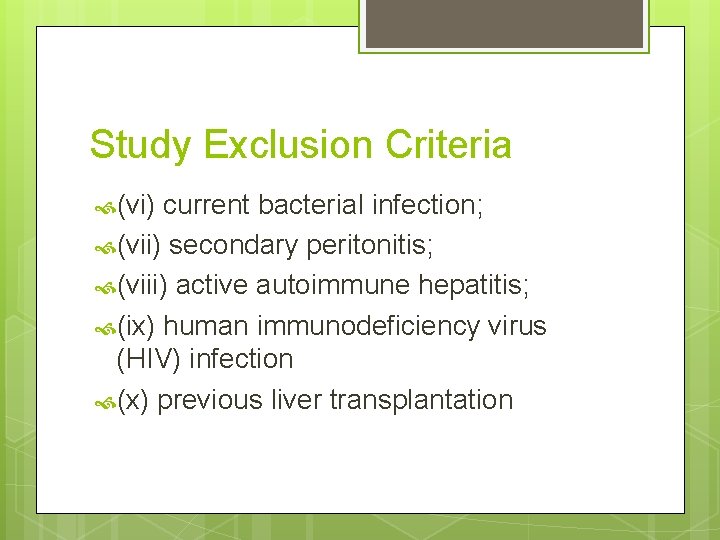
Study Exclusion Criteria (vi) current bacterial infection; (vii) secondary peritonitis; (viii) active autoimmune hepatitis; (ix) human immunodeficiency virus (HIV) infection (x) previous liver transplantation
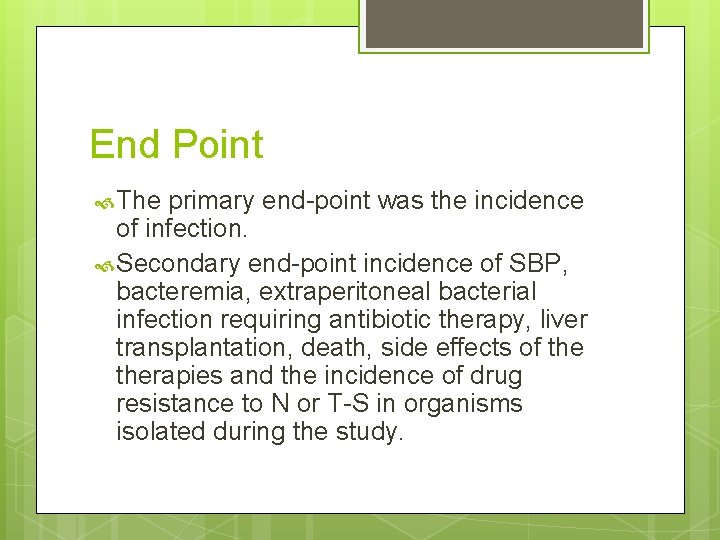
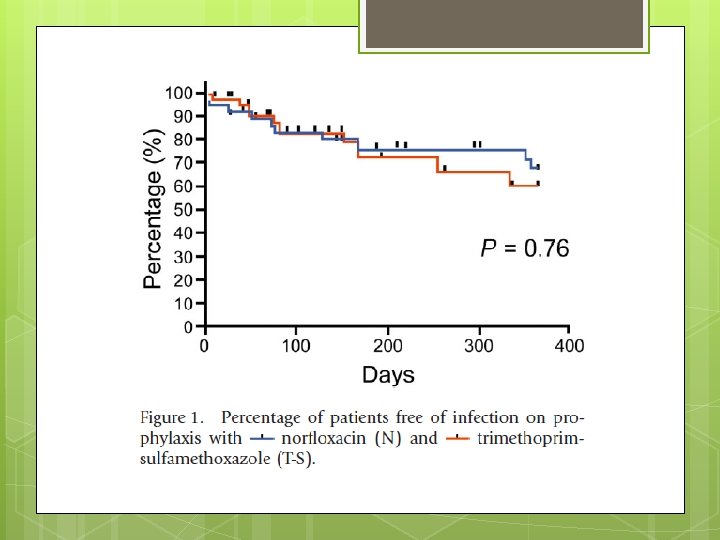
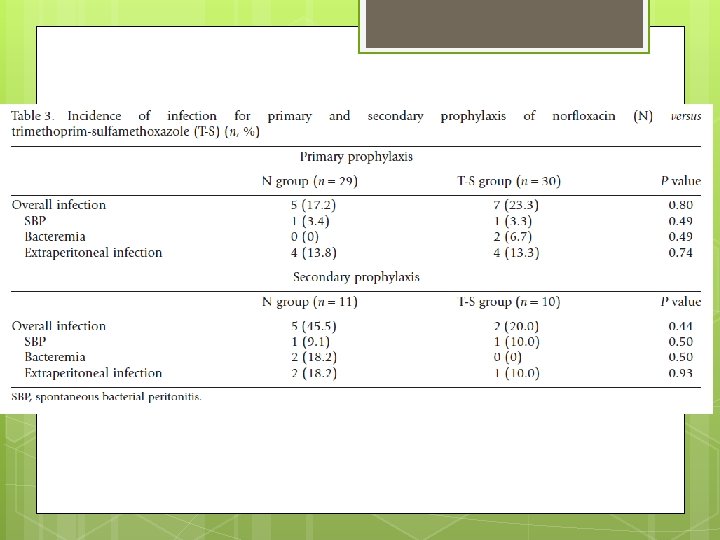
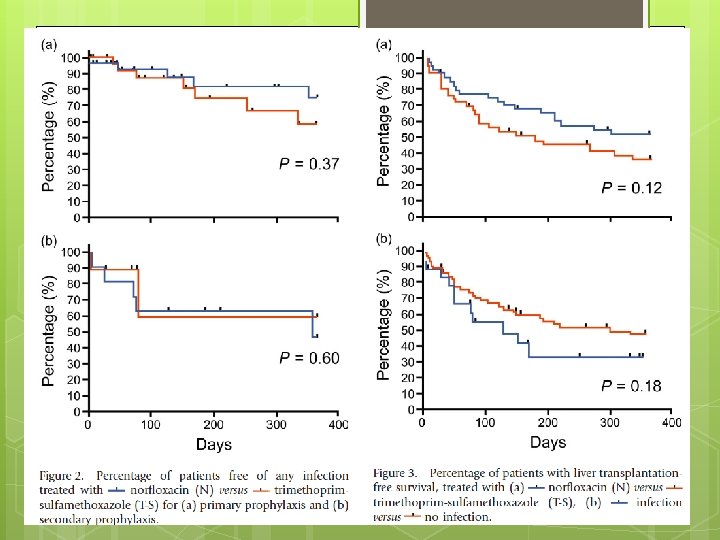
End Point The primary end-point was the incidence of infection. Secondary end-point incidence of SBP, bacteremia, extraperitoneal bacterial infection requiring antibiotic therapy, liver transplantation, death, side effects of therapies and the incidence of drug resistance to N or T-S in organisms isolated during the study.
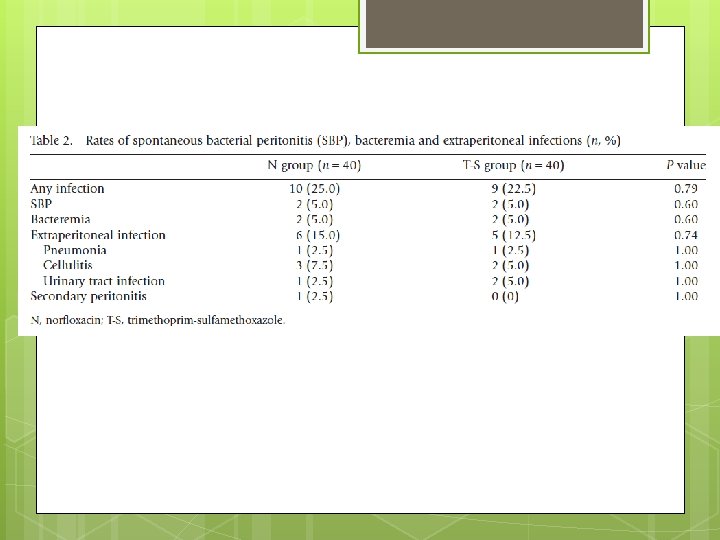

Resistance Pattern Five gram-positive, four gram-negative and one fungal culture were isolated. Two isolates (both gram negative organisms) from the T-S group were resistant to T-S but sensitive to N. Three organisms (all grampositive) from patients taking N showed in vitro resistance to N, with one of these being sensitive to T-S. None of the isolated gram-negative organisms were resistant to N.

Critical Appraisal

Advantages Randomized, controlled, non blinded trial. Stratifies with primary and secondary prophylaxis Kaplan Meier plot Repeat Paracentesis with proven culture data. Compares Bactrim Vs conventional fluoroquinolone to provide more options for prophylaxis.

Limitation Small sample size difficult to detect a difference. Especially for secondary prophylaxis. Non blinded to patient and investigator of medication.

Thank you
- Slides: 22