Saxenda Liraglutide SAMUEL GYAWUAMOATENG Indication Approval Saxenda is









![CO$T!!! 6 mg/m. L (3 m. L) : $256. 39 [1] CO$T!!! 6 mg/m. L (3 m. L) : $256. 39 [1]](https://slidetodoc.com/presentation_image_h/919fbf6b0aeafaa0d2df2b792e3c1c1a/image-11.jpg)

![Credits [1. ] Lexicomp http: //0 online. lexi. com. wildpac. wne. edu/lco/action/doc/retrieve/docid/patch_f/2144379 [2. ] Credits [1. ] Lexicomp http: //0 online. lexi. com. wildpac. wne. edu/lco/action/doc/retrieve/docid/patch_f/2144379 [2. ]](https://slidetodoc.com/presentation_image_h/919fbf6b0aeafaa0d2df2b792e3c1c1a/image-13.jpg)
- Slides: 13

Saxenda ( Liraglutide ) SAMUEL GYAWU-AMOATENG

Indication & Approval Saxenda, is FDA approved as a treatment option for chronic weight management in addition to a reduced-calorie diet and physical activity Drug was approved in December 23 rd 2014 It is approved in adults with BMI of 30 or greater (obesity) or adults with BMI of 27 or greater (overweight) with at least one weight-related condition such as HTN, T 2 DM, or Dyslipidemia.

Drug Class Saxenda is a glucagon-like peptide-1 (GLP-1) receptor agonist also known as incretin mimitic. Therefore is should not be used with other drugs belonging to the same class such as Victoza, for T 2 DM.

MOA A long acting analog of human glucagon-like peptide- (GLP-1) (an incretin hormone) which increases glucose-dependent insulin secretion, decreases inappropriate glucagon secretion, increase's B-cell growth/replication, slows gastric emptying, and decreases food intake. It’s administration results in decreases in hemoglobin A 1 c by ~1%

Dosage & Formulation Initial 0. 6 mg Sub. Q once daily for 1 week, increase by 0. 6 mg daily at weekly intervals to target the dose of 3 mg once daily. If 3 mg dose is not tolerated, d/c therapy since efficacy has not been established at lower doses. Evaluate changes in body weight 16 weeks after starting of therapy. d/c if at least 4% of baseline body weight loss has not been achieved. No dosage adjustments for renal or mild-to-severe hepatic impairment patient.

Administration & Storage Drug is administered Su. BQ in the upper arm, thigh or abdomen. Should not be injected IV, or IM. Can be administered w/o food at any time of the day Store at 36 to 46 degree F, prior to initial use, and same temp after initial use Do not freeze, discard if freezing occurs Discard pen 30 days after initial use

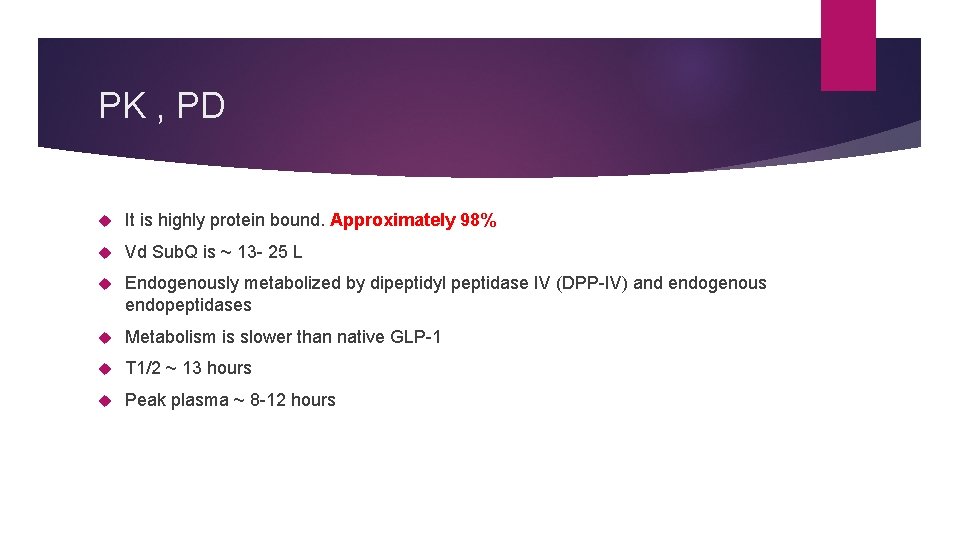
PK , PD It is highly protein bound. Approximately 98% Vd Sub. Q is ~ 13 - 25 L Endogenously metabolized by dipeptidyl peptidase IV (DPP-IV) and endogenous endopeptidases Metabolism is slower than native GLP-1 T 1/2 ~ 13 hours Peak plasma ~ 8 -12 hours

Side Effects and Adverse Effects Nausea, vomiting, diarrhea, tiredness, decrease appetite, headache Renal impairment Hypoglycemia, Gall bladder problems BBW : Thyroid Tumors: Dose dependent and treatment duration-dependent thyroid C-cell tumors.

Contraindications Saxenda is contraindicated in patients with: 1. Hypersensitive to liraglutide or an component of the product [2] 2. Personal or family history of medullary thyroid carcinoma [1, 2] 3. Personal or family history of multiple endocrine neoplasia syndrome type 2 (MEN 2) [1, 2] Pregnancy [1]

Usage
![COT 6 mgm L 3 m L 256 39 1 CO$T!!! 6 mg/m. L (3 m. L) : $256. 39 [1]](https://slidetodoc.com/presentation_image_h/919fbf6b0aeafaa0d2df2b792e3c1c1a/image-11.jpg)
CO$T!!! 6 mg/m. L (3 m. L) : $256. 39 [1]

Counseling Pearls & Monitoring Saxenda approved with a Risk Evaluation and Mitigation Strategy (REMS) program. Pregnancy Category X: Do not use if pregnant or plan on getting pregnant soon. If dose is missed for 3 days or more, contact doctor on how to restart Alternate injection site Monitor for signs & symptoms of hypoglycemia Monitor for signs and symptoms of pancreatitis, Monitor for mood changes or behavioral changes.
![Credits 1 Lexicomp http 0 online lexi com wildpac wne edulcoactiondocretrievedocidpatchf2144379 2 Credits [1. ] Lexicomp http: //0 online. lexi. com. wildpac. wne. edu/lco/action/doc/retrieve/docid/patch_f/2144379 [2. ]](https://slidetodoc.com/presentation_image_h/919fbf6b0aeafaa0d2df2b792e3c1c1a/image-13.jpg)
Credits [1. ] Lexicomp http: //0 online. lexi. com. wildpac. wne. edu/lco/action/doc/retrieve/docid/patch_f/2144379 [2. ] Micromedex http: //0 www. micromedexsolutions. com. wildpac. wne. edu/micromedex 2/librarian/ND_T/evidencexpert/ND_ PR/evidencexpert/CS/984 F 15/ND_App. Product/evidencexpert/DUPLICATIONSHIELDSYNC/CFB 7 0 A/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFAction. Id/evidencexpert. Int ermediate. To. Document. Link? doc. Id=2814&content. Set. Id=31&title=LIRAGLUTIDE&services. Title=LIR AGLUTIDE# [3. ] Liraglutide med guide