SAVE WATER SAVE LIFE 1 Plan In our




























- Slides: 40

SAVE WATER! SAVE LIFE! 1

Plan – In our presentation, we will follow the following plan: • 1) Water as the cradle of life • 2) Physical and chemical properties of water • 3) Pollution of water 2

Part 1 Water – the cradle of life 3

Water – the cradle of life • For proving the importance of water for the birth of life, we will take • • a look at the as known chemical evolution – the transition from inorganic materials to the simplest and fundamental form of life as we know it – the first cells. The cells are the basic structure, metabolic and functional unit of organisms. The evolution of life after the creation of cells is known as biological evolution, and we are not going into it in this presentation. 4

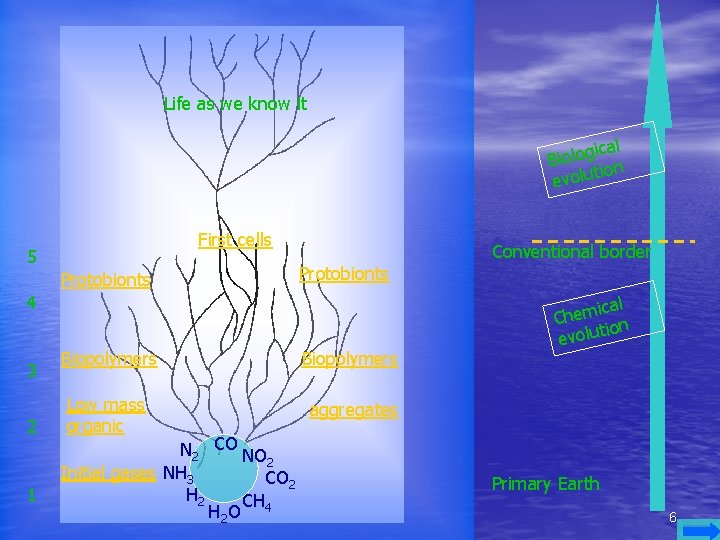
This tree shows the evolution of primary chemical elements until the forming of life as we know it. Notice that the greater part of evolution is before the formation of the first cells. 5

Life as we know it cal i g o l o Bi tion u l o v e First cells 5 Protobionts Conventional border Protobionts 4 3 2 1 Biopolymers Low mass organic aggregates N 2 CO NO 2 Initial gases NH 3 CO 2 H 2 CH H 2 O 4 ical m e h C tion evolu Primary Earth 6

Initial gases 1 stage Active volcanoes N 2 CO CO 2 H 2 NH 3 CH 4 NO 2 H 2 O • 1. Probably during the early stages of the Earth’s existence there were violent volcanic eruptions on the whole surface, congestion of the Earth’s crust and separation of a high amount of heat. The gases (water vapours, hydrogen, methane, ammonia), coming from the hot semi-fluid interior of the Earth, formed the atmosphere. It contained the basic materials for the formation of organic combinations. 7

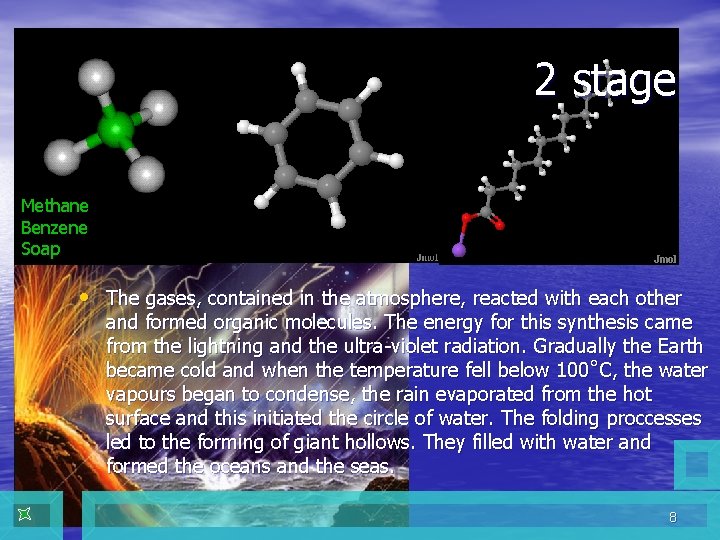
2 stage Methane Benzene Soap • The gases, contained in the atmosphere, reacted with each other and formed organic molecules. The energy for this synthesis came from the lightning and the ultra-violet radiation. Gradually the Earth became cold and when the temperature fell below 100˚С, the water vapours began to condense, the rain evaporated from the hot surface and this initiated the circle of water. The folding proccesses led to the forming of giant hollows. They filled with water and formed the oceans and the seas. 8

Miller’s experiment • An evidence for this is the experiment of Stanley Miller. He invented a device in which ammonia, methane, hydrogen and water vapours circulated and through the mixture passed an electric spark. During the circulation the water cooled and fell like a “rain”. After a week in the liquid were found amino acids. It is perfectly possible that the same processes happened in the atmosphere. 9

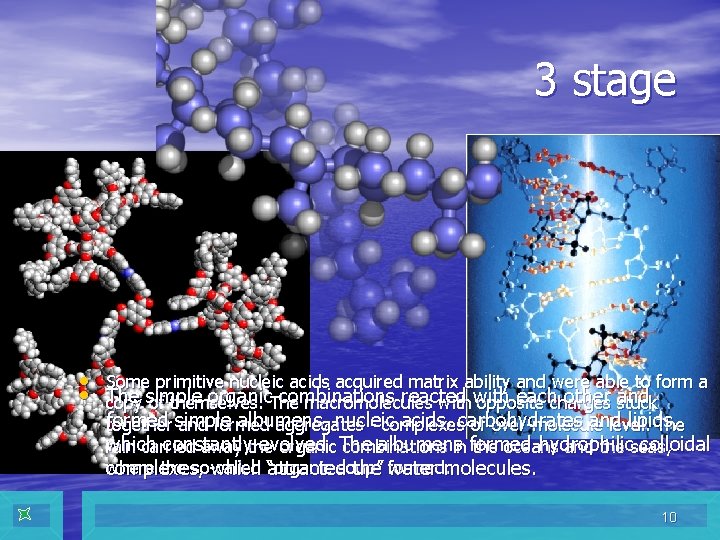
3 stage • Some primitive nucleic acids acquired matrix ability and were able to form a • The simple organic combinations reacted with each other and copy of themselves. The macromolecules with opposite charges stuck formed simple albumens, nucleic acids, carbohydrates and lipids, together and formed aggregates - complexes of over-molecule level. The which constantly evolved. The albumens formed hydrophilic colloidal rain carried away the organic combinations in the oceans and the seas, where the so-called “organic soup” formed. complexes, which attracted the water molecules. 10

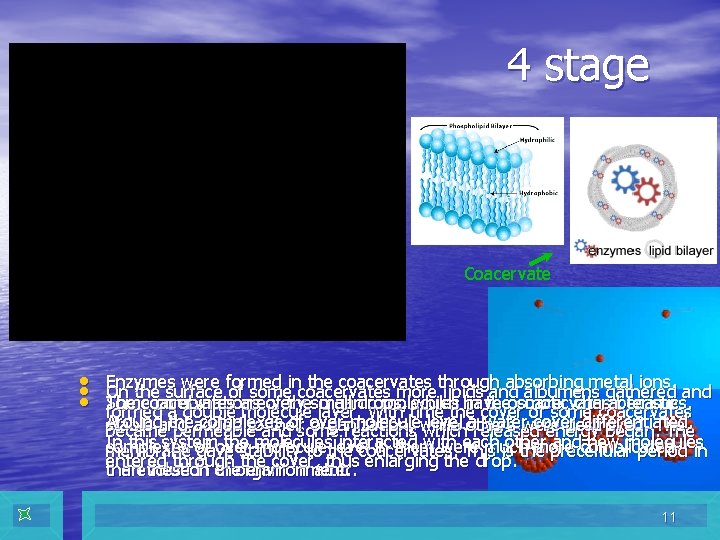
4 stage Coacervate • • • Enzymes were formed in the coacervates through absorbing metal ions. On the surface of some coacervates more lipids and albumens gathered and The coacervates are very small drops, which have osmotic characteristics. Some combinations of the organic molecules in the coacervates became formed a double molecule layer. With time the cover of some coacervates Around the complexes of over-molecule level a water cover differentiated. stable and added to their organization, while others were destroyed. The became permeable and some reactions which released energy began. The In this system the molecules interacted with each other and new molecules complexes of over-molecule level in them were much more complicated membrane gave stability to the coacervates. This is the precellular period in entered through the cover, thus enlarging the drop. the evolution of organic matter. than these in the environment. 11

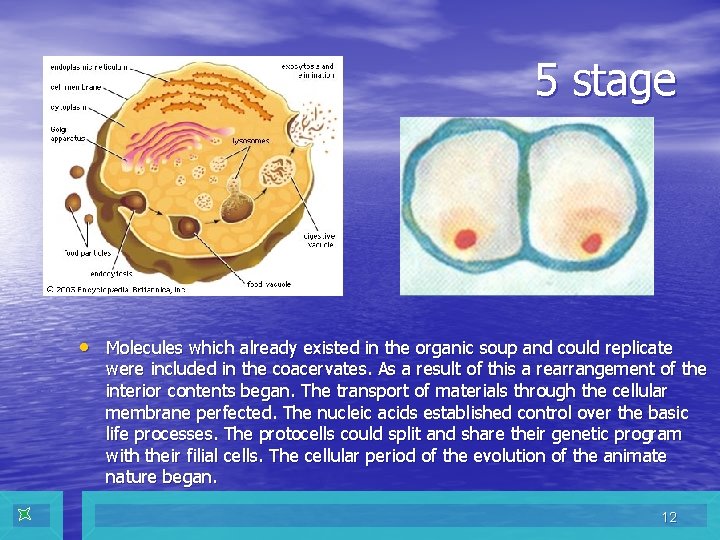
5 stage • Molecules which already existed in the organic soup and could replicate were included in the coacervates. As a result of this a rearrangement of the interior contents began. The transport of materials through the cellular membrane perfected. The nucleic acids established control over the basic life processes. The protocells could split and share their genetic program with their filial cells. The cellular period of the evolution of the animate nature began. 12

• … and life began … 13

Part 2 Properties of water 14

Water has no taste, no colour, no odour; it cannot be defined, art relished while ever mysterious. Not necessary to life, but rather life itself. It fills us with a gratification that exceeds the delight of the senses. ANTOINE DE SAINT-EXUPERY (1900 -1944), Wind, Sand, and Stars, 1939 15

Planet, dominated by water • We live on a planet that is dominated by water. • More than 70% of the Earth's surface is covered with this simple molecule. Scientists estimate that the hydrosphere contains about 1. 36 billion cubic kilometers of this substance mostly in the form of a liquid (water) that occupies topographic depressions on the Earth. The second most common form of the water molecule on our planet is ice. If all our planet's ice melted, sea-level would rise by about 70 meters. 16

• Water is also essential for life. Water is the major constituent of almost all life forms. Most animals and plants contain more than 60% water by volume. Without water life would probably never have developed on our planet. 17

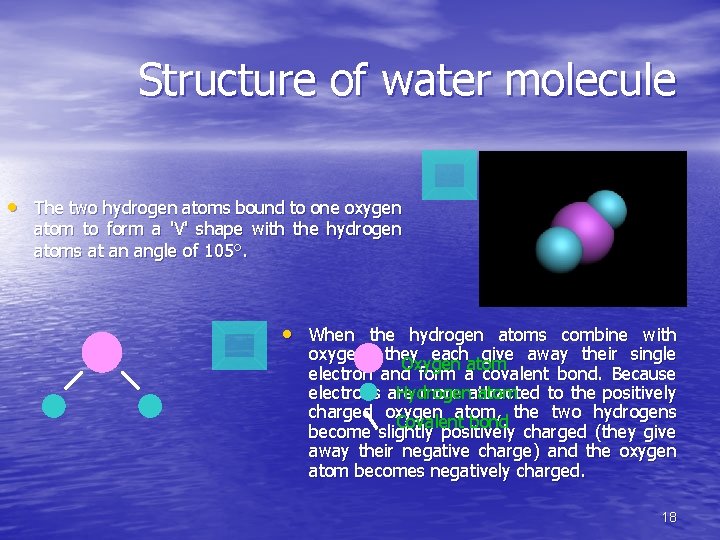
Structure of water molecule • The two hydrogen atoms bound to one oxygen atom to form a 'V' shape with the hydrogen atoms at an angle of 105°. • When the hydrogen atoms combine with oxygen, they each give away their single Oxygen atom electron and form a covalent bond. Because electrons are more attracted to the positively Hydrogen atom charged oxygen atom, the two hydrogens Covalent bond become slightly positively charged (they give away their negative charge) and the oxygen atom becomes negatively charged. 18

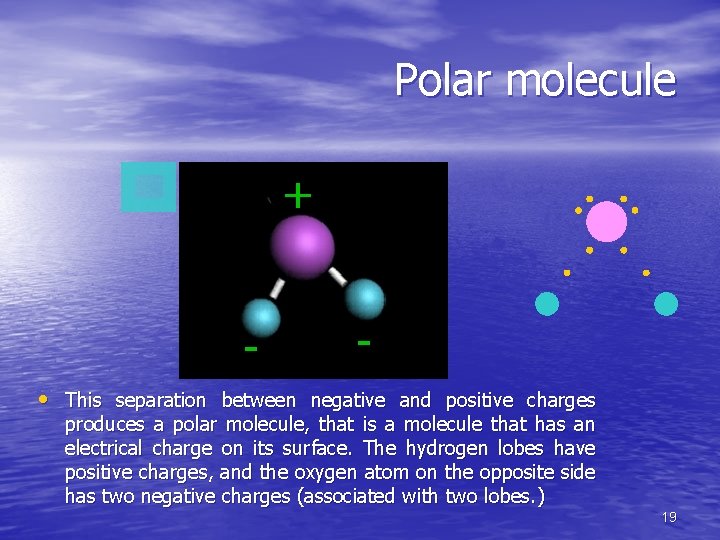
Polar molecule + - - • This separation between negative and positive charges produces a polar molecule, that is a molecule that has an electrical charge on its surface. The hydrogen lobes have positive charges, and the oxygen atom on the opposite side has two negative charges (associated with two lobes. ) 19

Hydrogen bond • Because they are polarized, two adjacent H 2 O molecules can form a linkage known as a hydrogen bond. • Hydrogen bonds have only about 1/20 the strength of a covalent bond. A hydrogen bond is therefore a weak chemical bond between a hydrogen atom in one polar molecule and a very electronegative atom of a second polar molecule. The hydrogen of one water molecule will be attracted to the oxygen of another water molecule. There are usually 48 molecules per group in liquid water. + - 20

• The water molecules form hydrogen bonds, giving shape to water as a liquid. Each single water molecule can form bonds with four other water molecules in a tetrahedral arrangement. Although these bonds are weak they lead to many other unique properties. 21

3 aggregate conditions • Water is found on Earth in all three forms: liquid, solid, gas, and is cycled though the water cycle. • Water is unique in that it is the only natural substance that is found in all three states -- liquid, solid (ice), and gas (steam) -- at the temperatures normally found on Earth. This is because the Earth is a very special planet with just the right range of temperatures and air pressures. Earth's water is constantly interacting, changing, and in movement. Water cycle. 22

Water properties • Water as a liquid has the following properties: • Water is a tasteless, odourless liquid at ambient temperature and pressure, and appears colourless, although it has its own intrinsic very light blue hue. Ice also appears colourless, and water vapor is essentially invisible as a gas. • Tasteless • Odourless • Colourless 23

Transparency • Water is transparent, and thus aquatic plants can live within the water because sunlight can reach them. Only strong UV light is slightly absorbed. 24

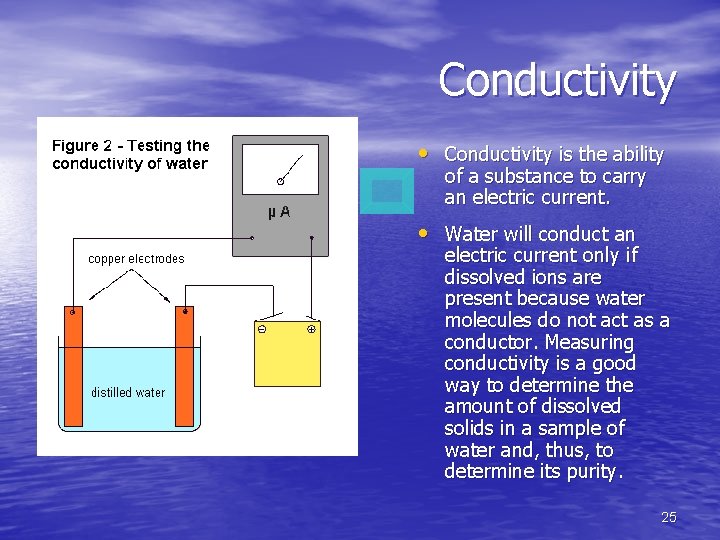
Conductivity • Conductivity is the ability of a substance to carry an electric current. • Water will conduct an electric current only if dissolved ions are present because water molecules do not act as a conductor. Measuring conductivity is a good way to determine the amount of dissolved solids in a sample of water and, thus, to determine its purity. 25

Water’s extension • Most liquids contract (get smaller) when they get colder. Water is different - it contracts until it reaches 4 C then it expands until it is solid. Solid water is less dense than liquid water because of this. • • • Water becomes even less dense upon If water worked like other liquids, The higher boiling point and melting then there would be no such thing freezing, expanding 9%. This causes an point of water can be explained by the as an ice berg, the ice in your soft unusual phenomenon: ice floats upon Hydrogen bonding accounts for the hydrogen bonds that help hold groups of drink would sink to the bottom of water, and so water organisms can live strength of fibres in wood. It also helps water molecules together. These the glass, and ponds would freeze inside a partly frozen pond because the explain some of the physical characteristics hydrogen bonds must be broken before from the bottom up! water on the bottom has a temperature The maximum of water. Water boils at 100°C and freezes water molecules can escape into the air. density of water is at 3. 98 °C of around 4 °C (39 °F). at 0°C. By comparison, sulfur dioxide, a This requires energy in the form of heat. (39. 16 °F). molecule of similar size, boils at 62°C and Molecules such as sulfur dioxide and freezes at -83°C. carbon dioxide do not have hydrogen bonds and, consequently, require less energy to boil. 26

Thermal capacity • Water has one of the highest heat capacities of all substances. • This means that it takes a great deal of heat energy to change the temperature of water compared to metals. • The large amount of water on Earth means that extreme temperature changes are rare on Earth compared to other planets. 27

Thermal buffer • Were it not for the high heat capacity of water, our bodies (which • also contain a large amount of water) would be subject to a great deal of temperature variation. By the two ways of defending against overheating (high heat capacity and high warmth of evaporation) water actually is an ideal medium for life and ensures safe temperature conditions in the organic systems. They also allow water to moderate Earth's climate by buffering large fluctuations in temperature. 28

Adhesion • Water molecules stick to each other. This is called cohesion. Water can also be attracted to other materials. This is called adhesion. • Water has a partial negative charge (σ-) near the oxygen atom due to the unshared pairs of electrons, and partial positive charges (σ+) near the hydrogen atoms. In water, this happens because the oxygen atom is more electronegative than the hydrogen atoms — that is, it has a stronger "pulling power" on the molecule's electrons, drawing them closer (along with their negative charge) and making the area around the oxygen atom more negative than the area around both of the 29 hydrogen atoms.

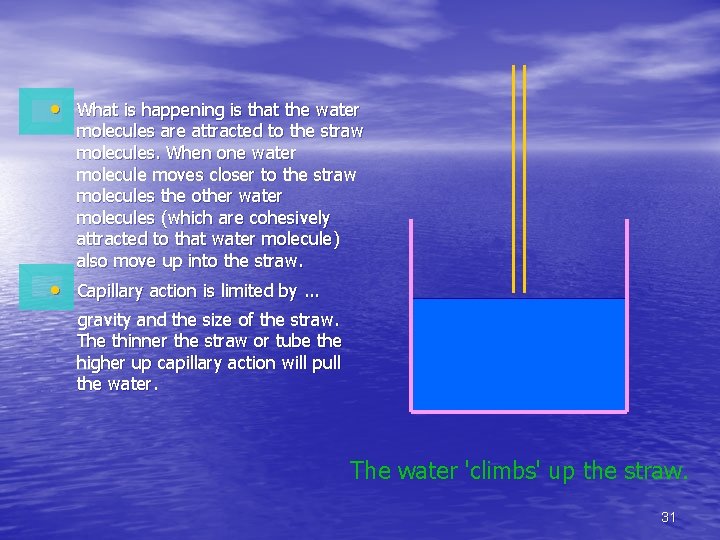
Capillary action • Capillary action is related • • to the adhesive properties of water. Plants take advantage of capillary action to pull water from the earth into themselves. You can see capillary action 'in action' by placing a straw into a glass of water. 30

• What is happening is that the water molecules are attracted to the straw molecules. When one water molecule moves closer to the straw molecules the other water molecules (which are cohesively attracted to that water molecule) also move up into the straw. • Capillary action is limited by. . . gravity and the size of the straw. The thinner the straw or tube the higher up capillary action will pull the water. The water 'climbs' up the straw. 31

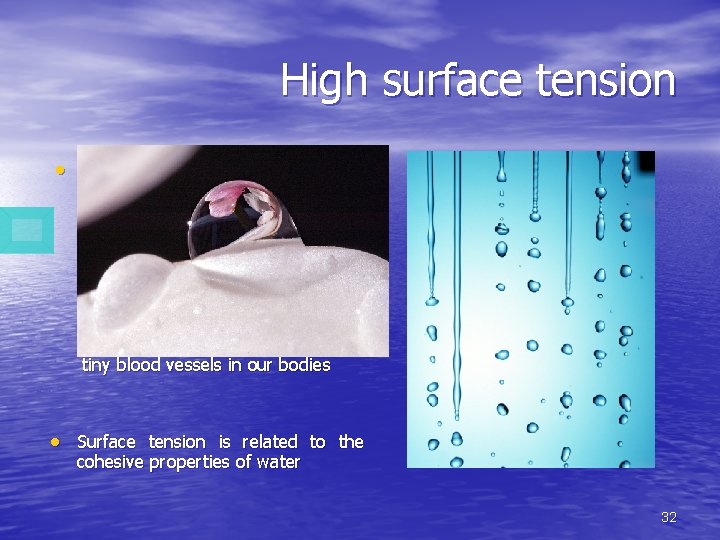
High surface tension Water has a very high surface tension. In other words, water is sticky and elastic, and tends to clump together in drops rather than spread out in a thin film. Surface tension is responsible for capillary action, which allows water (and its dissolved substances) to move through the roots of plants and through the tiny blood vessels in our bodies Surface tension is related to the cohesive properties of water 32

• This phenomenon also causes water to stick to the sides of vertical structures despite gravity's downward pull. Water's high surface tension allows for the formation of water droplets and waves, allows plants to move water (and dissolved nutrients) from their roots to their leaves, and the movement of blood through tiny vessels in the bodies of some animals. This insect walks on the water’s surface 33

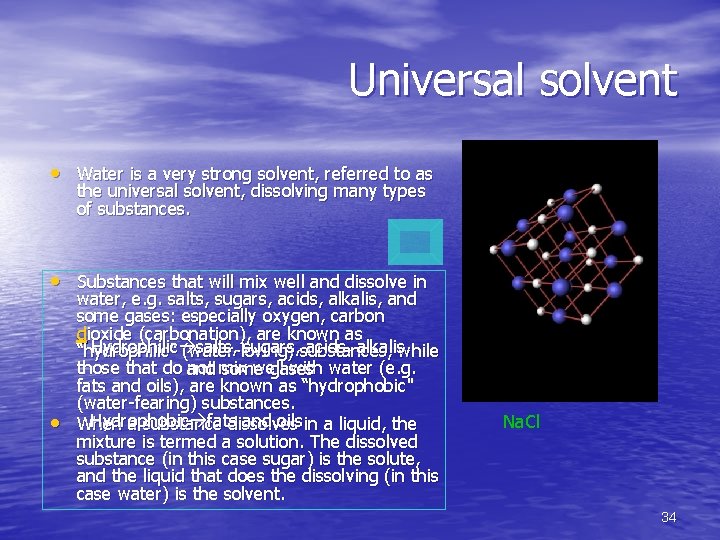
Universal solvent • Water is a very strong solvent, referred to as the universal solvent, dissolving many types of substances. • Substances that will mix well and dissolve in • water, e. g. salts, sugars, acids, alkalis, and some gases: especially oxygen, carbon dioxide (carbonation), are known as Hydrophilic salts, sugars, acids, alkalis, “hydrophilic" (water-loving) substances, while those that do not mix well with water (e. g. and some gases fats and oils), are known as “hydrophobic" (water-fearing) substances. Hydrophobic fats and oils When a substance dissolves in a liquid, the mixture is termed a solution. The dissolved substance (in this case sugar) is the solute, and the liquid that does the dissolving (in this case water) is the solvent. Na. Cl 34

• They dissolve in water, because their molecules separate from each other, each becoming surrounded by water molecules. 35

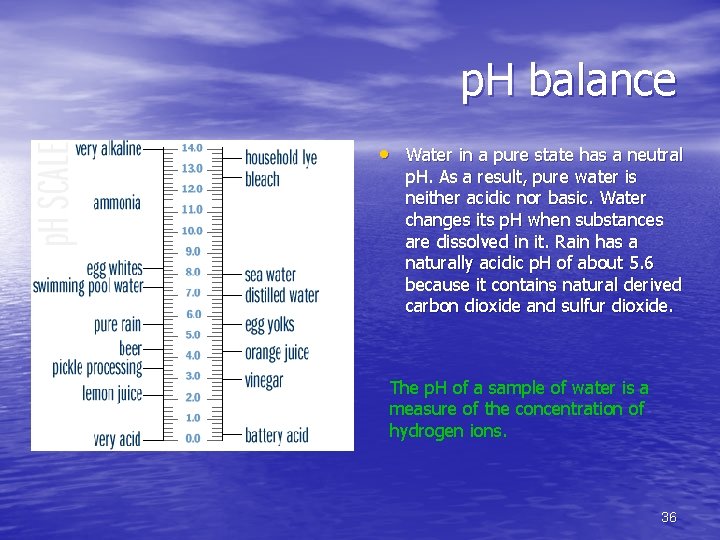
p. H balance • Water in a pure state has a neutral p. H. As a result, pure water is neither acidic nor basic. Water changes its p. H when substances are dissolved in it. Rain has a naturally acidic p. H of about 5. 6 because it contains natural derived carbon dioxide and sulfur dioxide. The p. H of a sample of water is a measure of the concentration of hydrogen ions. 36

Biochemical reactions • Water is a regulator of biochemical reactions. All of them start and • complete by direct or indirect reaction with the water molecules in the cell. The speed of the biochemical processes is reduced by the reduction of water content under some limits. With tremendous odds against unicellular organisms, they exude water, as a result of which the cytosol is condensed and the vital processes are reduced to a minimum. This state of organisms is called 'vita minima' or anabiosis. At optimal living conditions the amount of water in the organisms gets back to normal and the constitution is restored to its normal active state. 37

Afterword • Water is closely connected not only with the birth of life, but with its evolution as well. It has always been a limiting factor. The lack of water is one of the main forces controlling the natural selection. All terrestrial organisms take water and preserve it by some means or other. Clear examples can easily be found among desert ones such as cacti and camels. • Once the unique role of water in the living systems was expressed by a prominent biochemist Szent-Dyerdyi in the phrase: “Water is not only a mother, but a matrix of life”. That is why everybody should take thought of it. 38

39

Conclusion : • Save water! • Save life! Author: Diana Sofronieva 40