SAPIEN Program Update Next Generation Improvements and Design




- Slides: 25

SAPIEN Program Update, Next Generation Improvements, and Design Features Martyn Thomas VP of Medical Affairs Transcatheter Heart Valves Edwards Lifesciences

Disclosures Martyn Thomas MD Employee and Stockholder Edwards Lifesciences

Outline • Data from 2016 for the SAPIEN programme • Next generation device and rationale • Ongoing and planned future trials using the SAPIEN platforms

High Quality Sessions at EACTS In Europe…

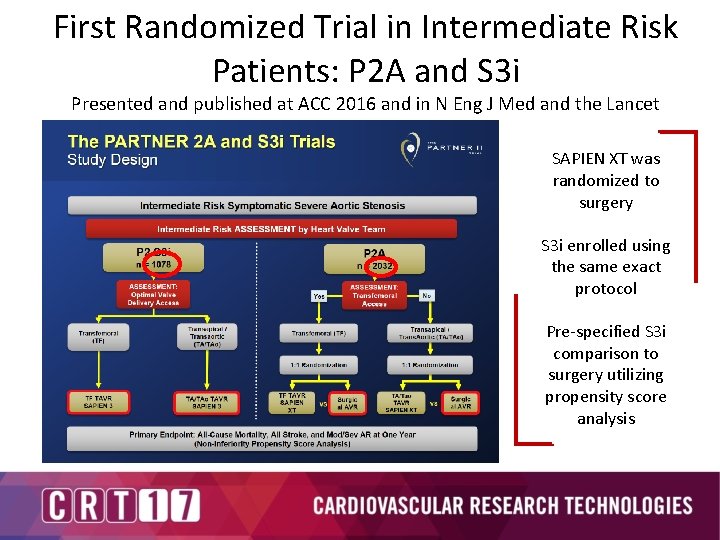
First Randomized Trial in Intermediate Risk Patients: P 2 A and S 3 i Presented and published at ACC 2016 and in N Eng J Med and the Lancet SAPIEN XT was randomized to surgery S 3 i enrolled using the same exact protocol Pre-specified S 3 i comparison to surgery utilizing propensity score analysis

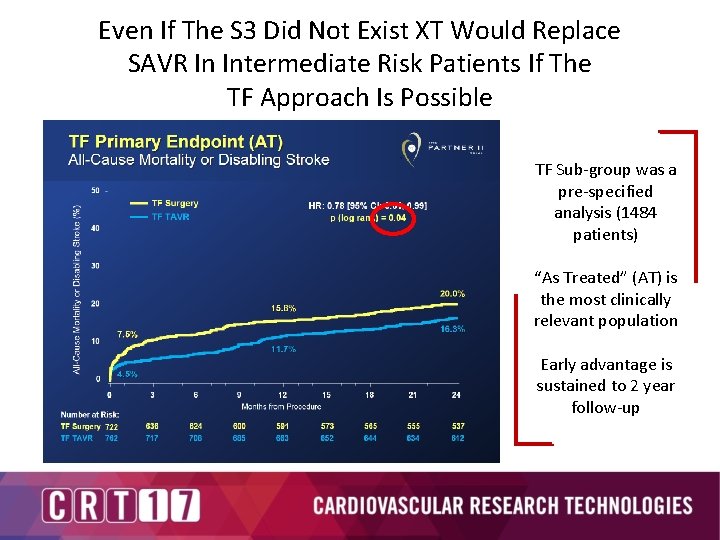
Even If The S 3 Did Not Exist XT Would Replace SAVR In Intermediate Risk Patients If The TF Approach Is Possible TF Sub-group was a pre-specified analysis (1484 patients) “As Treated” (AT) is the most clinically relevant population Early advantage is sustained to 2 year follow-up

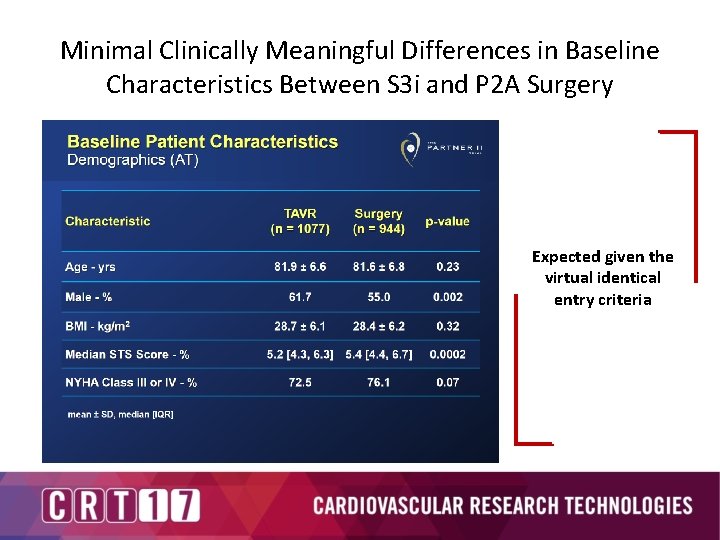
Minimal Clinically Meaningful Differences in Baseline Characteristics Between S 3 i and P 2 A Surgery Expected given the virtual identical entry criteria

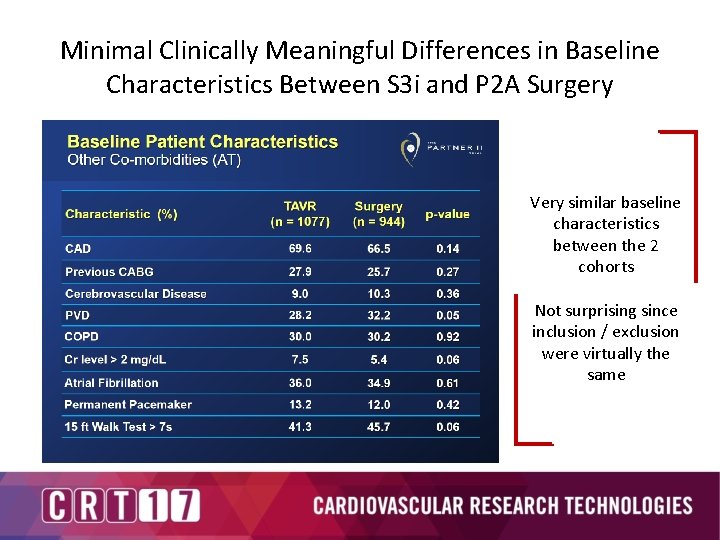
Minimal Clinically Meaningful Differences in Baseline Characteristics Between S 3 i and P 2 A Surgery Very similar baseline characteristics between the 2 cohorts Not surprising since inclusion / exclusion were virtually the same

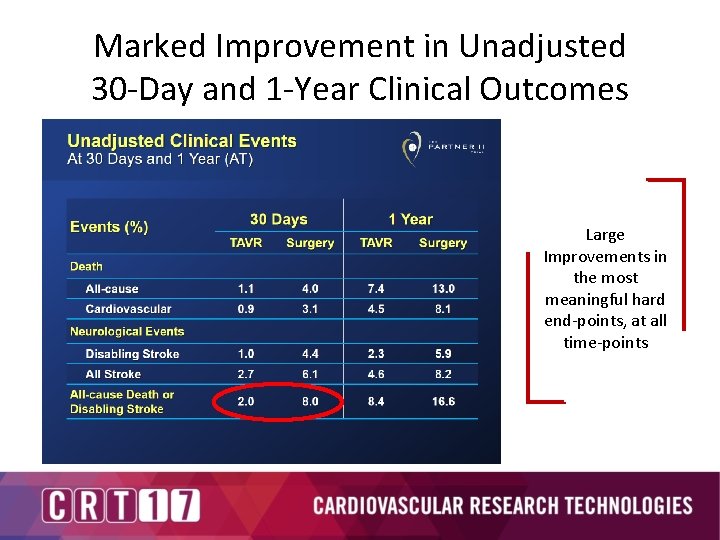
Marked Improvement in Unadjusted 30 -Day and 1 -Year Clinical Outcomes Large Improvements in the most meaningful hard end-points, at all time-points

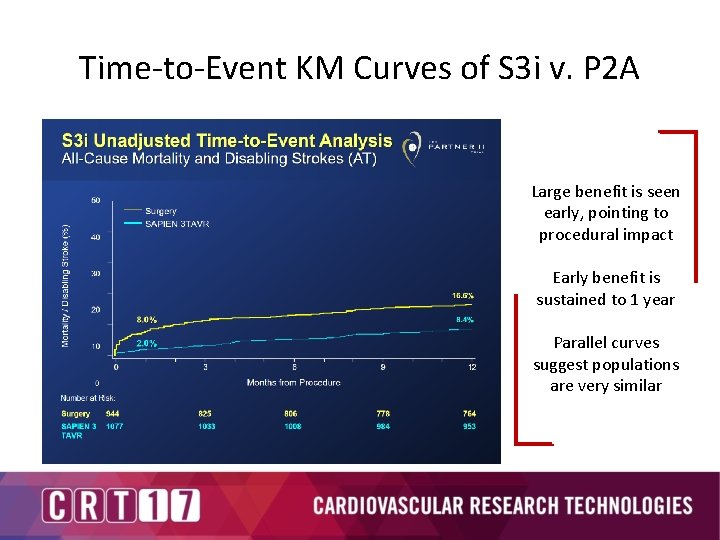
Time-to-Event KM Curves of S 3 i v. P 2 A Large benefit is seen early, pointing to procedural impact Early benefit is sustained to 1 year Parallel curves suggest populations are very similar

FDA Mandated methodology. S 3 i-PIIA SAVR Propensity Model Variables. • Logistic regression model performed on the following variables to generate propensity score. • Performed by independent statistician. Only had Group A and Group B with no outcomes data. NB: NOT MATCHING……………QUINTILE METHODOLOGY • • • Age Sex NYHA Class Angina Class BMI Native Annular Diameter Prior Stroke Porcelain Aorta COPD Diabetes Mellitus • Renal Insufficiency • • • Peripheral Vascular Disease Prior PCI Cardiomyopathy Carotid Disease Coronary Artery Disease Previous or current smoker Hypertension Prior Myocardial Infarction Pacemaker Prior Aortic Valvuloplasty Prior CABG

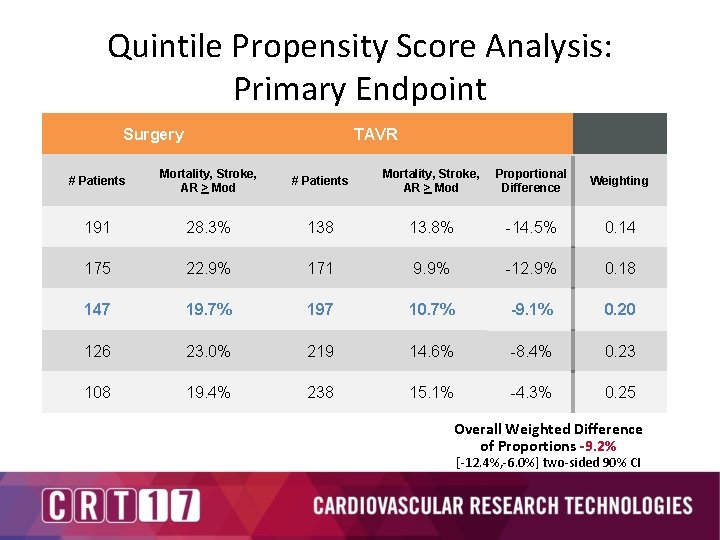
Quintile Propensity Score Analysis: Primary Endpoint Surgery TAVR # Patients Mortality, Stroke, AR > Mod Proportional Difference Weighting 191 28. 3% 138 13. 8% -14. 5% 0. 14 175 22. 9% 171 9. 9% -12. 9% 0. 18 147 19. 7% 197 10. 7% -9. 1% 0. 20 126 23. 0% 219 14. 6% -8. 4% 0. 23 108 19. 4% 238 15. 1% -4. 3% 0. 25 Overall Weighted Difference of Proportions -9. 2% [-12. 4%, -6. 0%] two-sided 90% CI

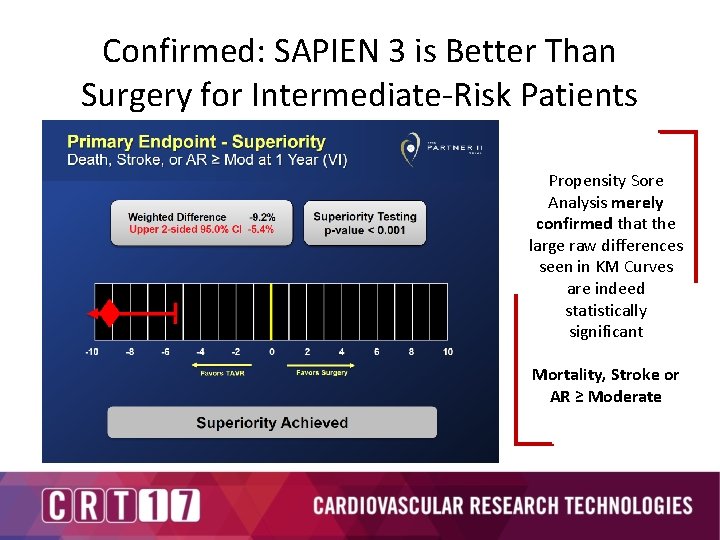
Confirmed: SAPIEN 3 is Better Than Surgery for Intermediate-Risk Patients Propensity Sore Analysis merely confirmed that the large raw differences seen in KM Curves are indeed statistically significant Mortality, Stroke or AR ≥ Moderate

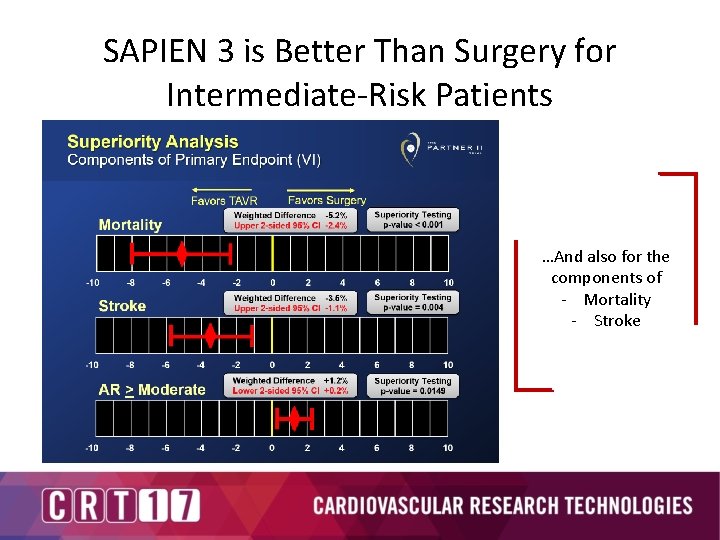
SAPIEN 3 is Better Than Surgery for Intermediate-Risk Patients …And also for the components of - Mortality - Stroke

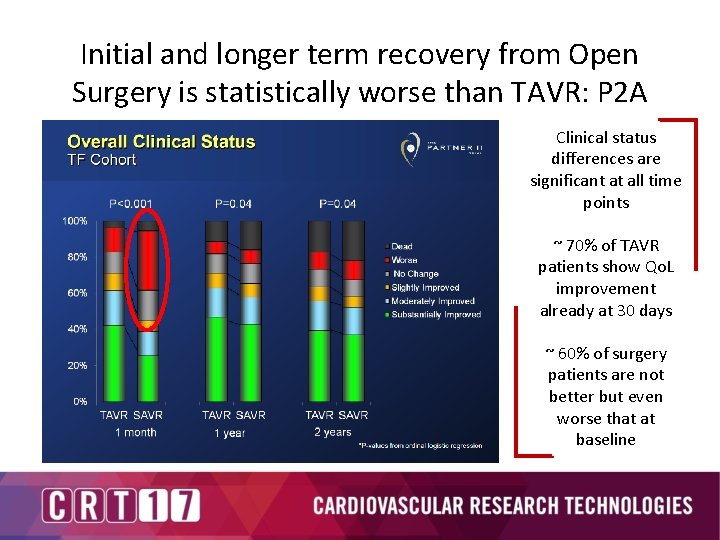
Initial and longer term recovery from Open Surgery is statistically worse than TAVR: P 2 A Clinical status differences are significant at all time points ~ 70% of TAVR patients show Qo. L improvement already at 30 days ~ 60% of surgery patients are not better but even worse that at baseline

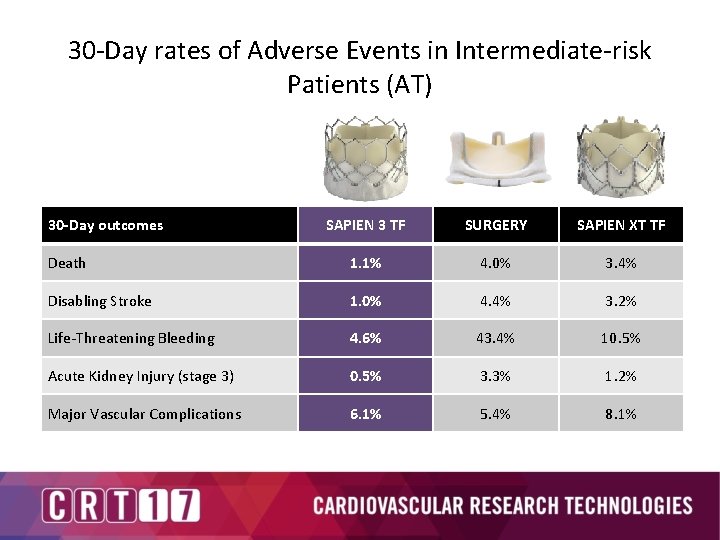
30 -Day rates of Adverse Events in Intermediate-risk Patients (AT) 30 -Day outcomes SAPIEN 3 TF SURGERY SAPIEN XT TF Death 1. 1% 4. 0% 3. 4% Disabling Stroke 1. 0% 4. 4% 3. 2% Life-Threatening Bleeding 4. 6% 43. 4% 10. 5% Acute Kidney Injury (stage 3) 0. 5% 3. 3% 1. 2% Major Vascular Complications 6. 1% 5. 4% 8. 1%

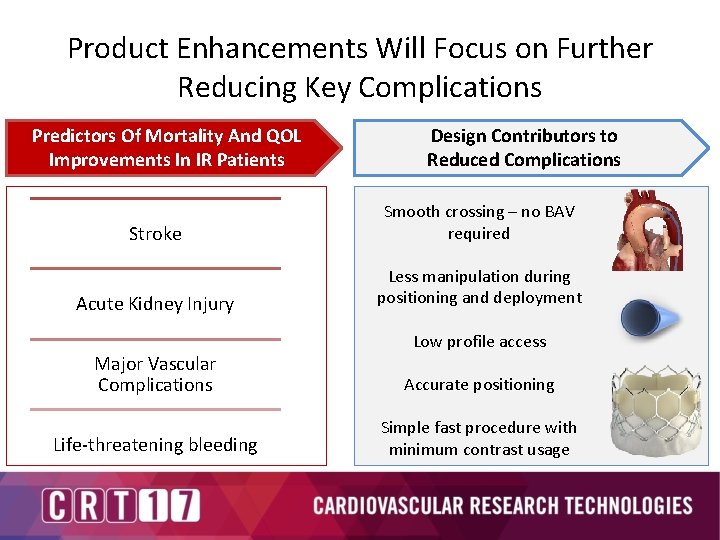
Product Enhancements Will Focus on Further Reducing Key Complications Predictors Of Mortality And QOL Improvements In IR Patients Design Contributors to Reduced Complications Stroke Smooth crossing – no BAV required Acute Kidney Injury Less manipulation during positioning and deployment Low profile access Major Vascular Complications Accurate positioning Life-threatening bleeding Simple fast procedure with minimum contrast usage

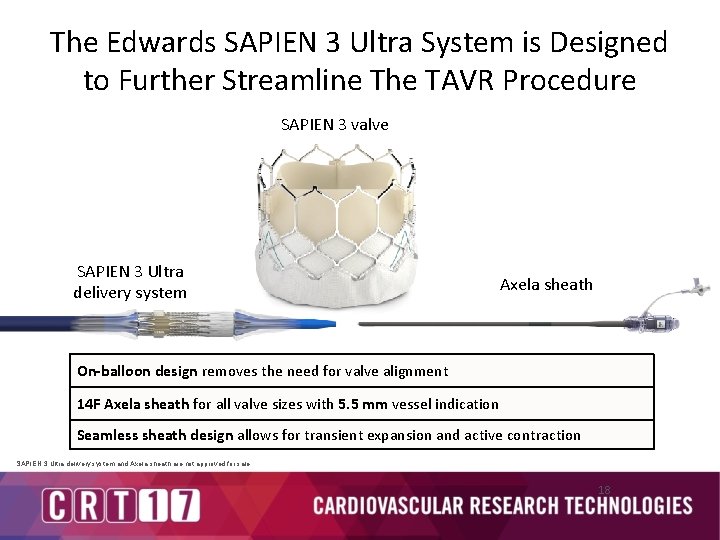
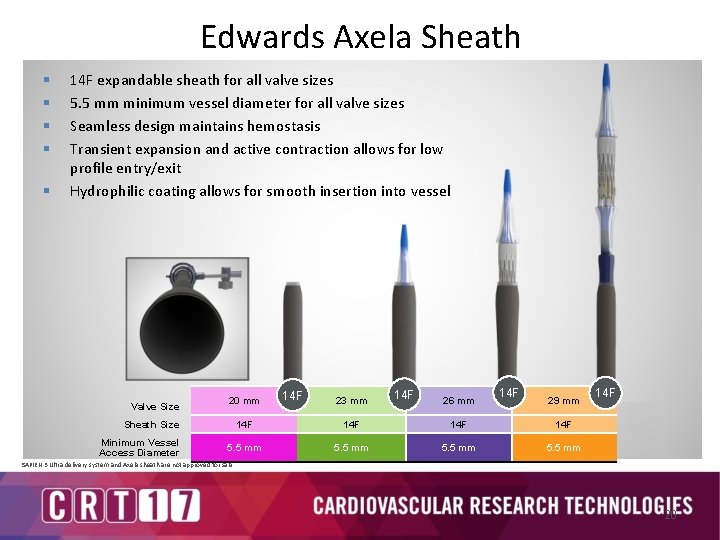
The Edwards SAPIEN 3 Ultra System is Designed to Further Streamline The TAVR Procedure SAPIEN 3 valve SAPIEN 3 Ultra delivery system Axela sheath On-balloon design removes the need for valve alignment 14 F Axela sheath for all valve sizes with 5. 5 mm vessel indication Seamless sheath design allows for transient expansion and active contraction SAPIEN 3 Ultra delivery system and Axela sheath are not approved for sale. 18

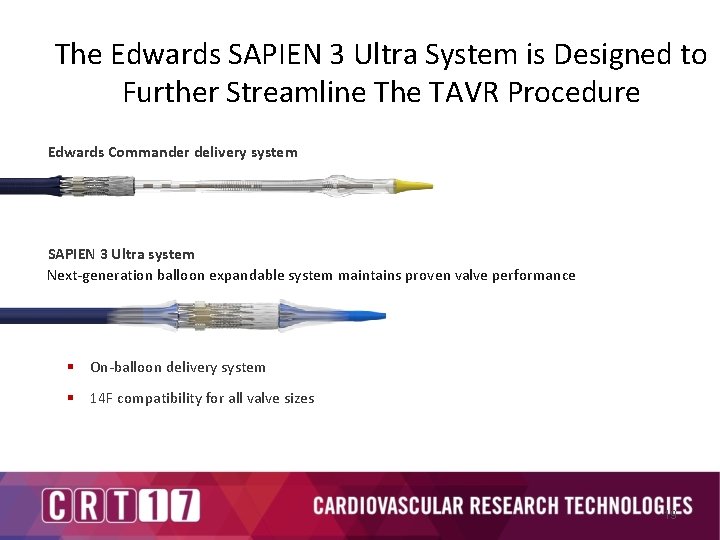
The Edwards SAPIEN 3 Ultra System is Designed to Further Streamline The TAVR Procedure Edwards Commander delivery system SAPIEN 3 Ultra system Next-generation balloon expandable system maintains proven valve performance § On-balloon delivery system § 14 F compatibility for all valve sizes 19

Edwards Axela Sheath § § § 14 F expandable sheath for all valve sizes 5. 5 mm minimum vessel diameter for all valve sizes Seamless design maintains hemostasis Transient expansion and active contraction allows for low profile entry/exit Hydrophilic coating allows for smooth insertion into vessel Valve Size 20 mm Sheath Size Minimum Vessel Access Diameter 14 F 23 mm 14 F 26 mm 14 F 29 mm 14 F 14 F 5. 5 mm 14 F SAPIEN 3 Ultra delivery system and Axela sheath are not approved for sale. 20

• Ongoing and future clinical studies in the SAPIEN programme

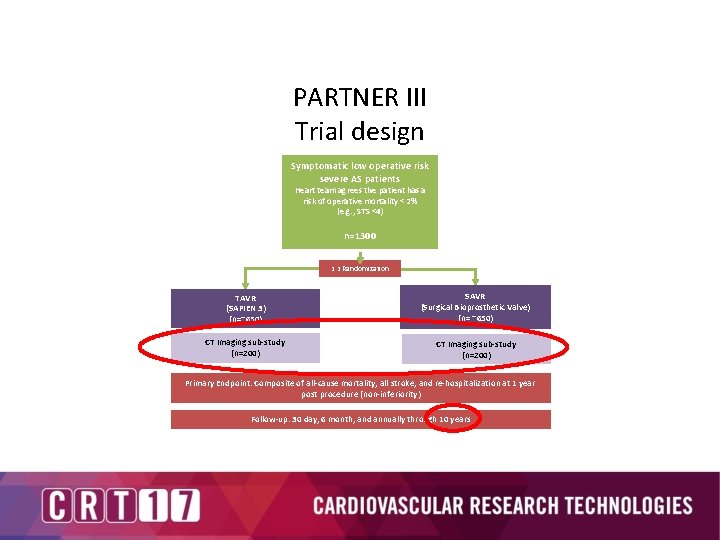
PARTNER III Trial design Symptomatic low operative risk severe AS patients Heart team agrees the patient has a risk of operative mortality < 2% (e. g. , STS <4) n=1300 1: 1 Randomization TAVR (SAPIEN 3) (n=~650) SAVR (Surgical Bioprosthetic Valve) (n= ~650) CT Imaging sub-study (n=200) Primary Endpoint: Composite of all-cause mortality, all stroke, and re-hospitalization at 1 year post procedure (non-inferiority) Follow-up: 30 day, 6 month, and annually through 10 years

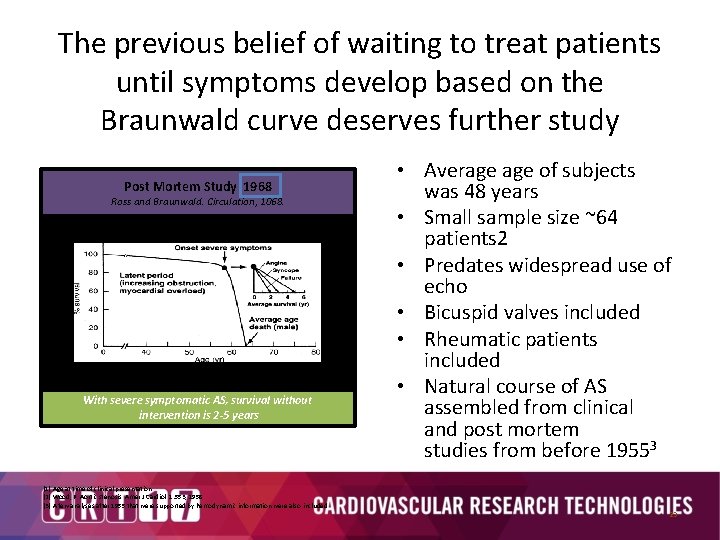
The previous belief of waiting to treat patients until symptoms develop based on the Braunwald curve deserves further study Post Mortem Study 1968 Ross and Braunwald. Circulation, 1068. Valvular Aortic Stenosis in Adults Average Course (Post Mortem Data) With severe symptomatic AS, survival without intervention is 2 -5 years • Average of subjects was 48 years • Small sample size ~64 patients 2 • Predates widespread use of echo • Bicuspid valves included • Rheumatic patients included • Natural course of AS assembled from clinical and post mortem studies from before 19553 (1) Age at time of clinical presentation (2) Wood, P. Aortic stenosis. Amer J Cardiol. 1: 553, 1958. (3) A few analyses after 1955 that were supported by hemodynamic information were also included 23

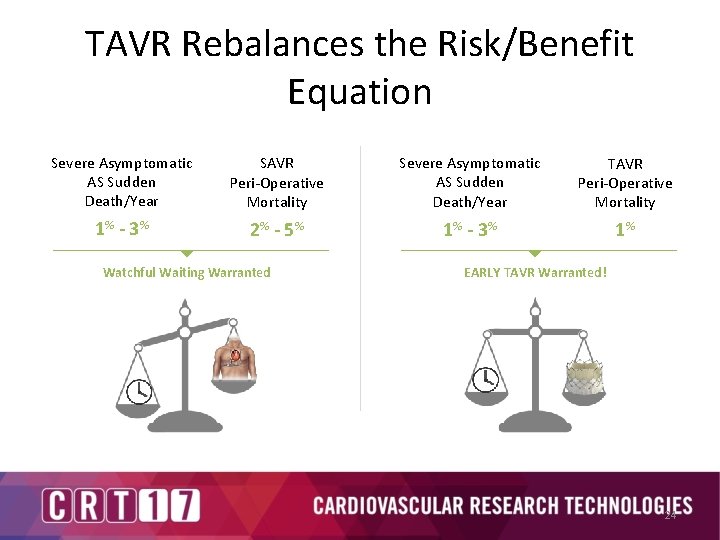
TAVR Rebalances the Risk/Benefit Equation Severe Asymptomatic AS Sudden Death/Year SAVR Peri-Operative Mortality Severe Asymptomatic AS Sudden Death/Year TAVR Peri-Operative Mortality 1% - 3 % 2% - 5 % 1% - 3 % 1% Watchful Waiting Warranted EARLY TAVR Warranted! 24

Conclusions • The future of the SAPIEN family of valves and delivery systems is bright. • Ultra and Axela will further simplify the procedure and should further reduce complications that impact on 1 year mortality. • The next generation device following this is well into development but TOP SECRET!! • Current and future trials should allow TAVR to be an option for Heart Teams for all severe AS patients.