Santa Rosa Junior College Physics Department Spring 2006











- Slides: 17

Santa Rosa Junior College Physics Department Spring 2006, Physics 4 D Instructor: Younes Ataiiyan By: Fermin Pureco, David Henderson, and Ivan Sanchez

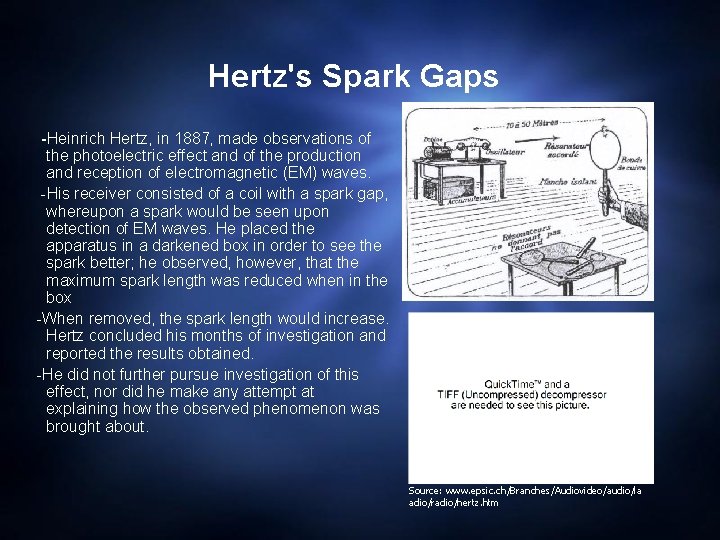
Hertz's Spark Gaps -Heinrich Hertz, in 1887, made observations of the photoelectric effect and of the production and reception of electromagnetic (EM) waves. -His receiver consisted of a coil with a spark gap, whereupon a spark would be seen upon detection of EM waves. He placed the apparatus in a darkened box in order to see the spark better; he observed, however, that the maximum spark length was reduced when in the box -When removed, the spark length would increase. Hertz concluded his months of investigation and reported the results obtained. -He did not further pursue investigation of this effect, nor did he make any attempt at explaining how the observed phenomenon was brought about. Source: www. epsic. ch/Branches/Audiovideo/audio/la adio/radio/hertz. htm

Joseph John Thomson’s CRT -In 1899, Joseph John Thomson investigated ultraviolet light in Crookes tubes. -In the research, Thomson enclosed a metal plate (a cathode) in a vacuum tube, and exposed it to high frequency radiation. It was thought that the oscillating electromagnetic fields caused the atoms' field to resonate and, after reaching a certain amplitude, caused a subatomic "corpuscle" to be emitted, and current to be detected. -The amount of this current varied with the intensity and color of the radiation. Larger Source: www. luminet. net/~wenonah/history/rife. htm radiation intensity or frequency would produce more current.

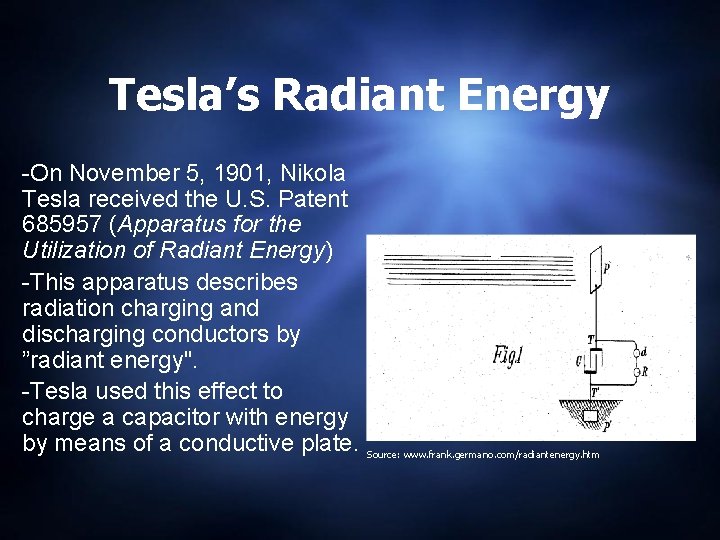
Tesla’s Radiant Energy -On November 5, 1901, Nikola Tesla received the U. S. Patent 685957 (Apparatus for the Utilization of Radiant Energy) -This apparatus describes radiation charging and discharging conductors by ”radiant energy". -Tesla used this effect to charge a capacitor with energy by means of a conductive plate. Source: www. frank. germano. com/radiantenergy. htm

Lenard’s Observations -In 1902, Philipp von Lenard observed the variation in electron energy with light frequency. He used a powerful electric arc lamp which enabled him to investigate large changes in intensity, and had sufficient power to enable him to investigate the variation of potential with light frequency. -His experiment directly measured potentials, not electron kinetic energy: he found the electron energy by relating it to the maximum stopping potential (voltage) in a phototube. He found that the calculated maximum electron kinetic energy is determined by the frequency of the light. -For example, an increase in frequency results in an increase in the maximum kinetic energy calculated for an electron upon liberation - ultraviolet radiation would require a higher applied stopping potential to stop current in a phototube than blue light. However Lenard's results were qualitative rather than quantitative because of the difficulty in performing the experiments -The current emitted by the surface was determined by the light's intensity, or brightness: doubling the intensity of the light doubled the number of electrons emitted from the surface. Source: http: //nobelprize. org/physics/ laureates/1905/lenard-bio. html

Photoelectric Effect Theory

Concerning an Heuristic Point of View Toward the Emission and Transformation of Light �”It seems to me that the observations associated with blackbody radiation, fluorescence, the production of cathode rays by ultraviolet light, and other related phenomena connected with the emission or transformation of light are more readily understood if one assumes that the energy of light is discontinuously distributed in space. In accordance with the assumption to be considered here, the energy of a light ray spreading out from a point source is not continuously distributed over an increasing space but consists of a finite number of energy quanta which are localized at points in space, which move without dividing, and which can only be produced and absorbed as complete units. ” A. Einstein, Ann. Phys. 17, 132 1905

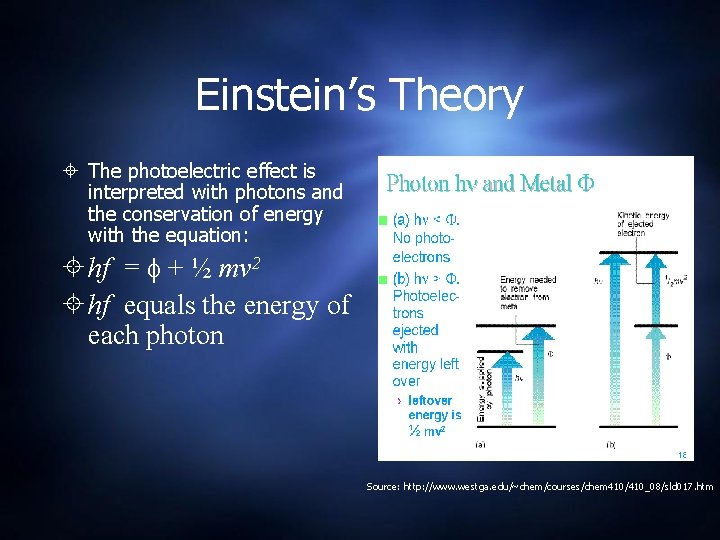
Einstein’s Theory The photoelectric effect is interpreted with photons and the conservation of energy with the equation: hf = + ½ mv 2 hf equals the energy of each photon Source: http: //www. westga. edu/~chem/courses/chem 410/410_08/sld 017. htm

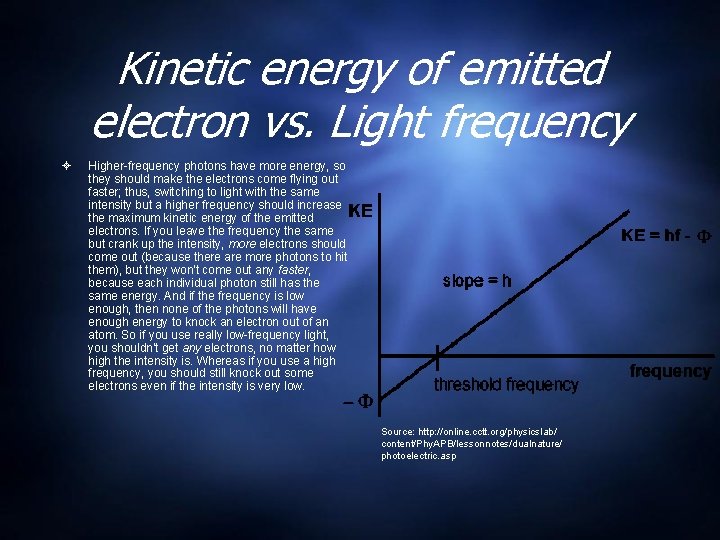
Kinetic energy of emitted electron vs. Light frequency Higher-frequency photons have more energy, so they should make the electrons come flying out faster; thus, switching to light with the same intensity but a higher frequency should increase the maximum kinetic energy of the emitted electrons. If you leave the frequency the same but crank up the intensity, more electrons should come out (because there are more photons to hit them), but they won't come out any faster, because each individual photon still has the same energy. And if the frequency is low enough, then none of the photons will have enough energy to knock an electron out of an atom. So if you use really low-frequency light, you shouldn't get any electrons, no matter how high the intensity is. Whereas if you use a high frequency, you should still knock out some electrons even if the intensity is very low. Source: http: //online. cctt. org/physicslab/ content/Phy. APB/lessonnotes/dualnature/ photoelectric. asp

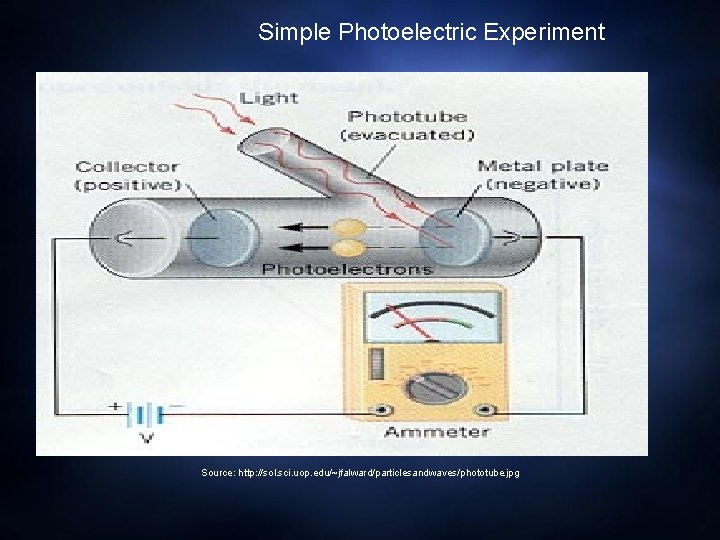
Simple Photoelectric Experiment Source: http: //sol. sci. uop. edu/~jfalward/particlesandwaves/phototube. jpg

Photoelectric Effect Applications

Applications The Photoelectric effect has numerous applications, for example night vision devices take advantage of the effect. Photons entering the device strike a plate which causes electrons to be emitted, these pass through a disk consisting of millions of channels, the current through these are amplified and directed towards a fluorescent screen which glows when electrons hit it. Image converters, image intensifiers, television camera tubes, and image storage tubes also take advantage of the point-by-point emission of the photocathode. In these devices an optical image incident on a semitransparent photocathode is used to transform the light image into an “electron image. ” The electrons released by each element of the photoemitter are focused by an electron-optical device onto a fluorescent screen, reconverting it in the process again into an optical image

Applications: Night Vision Device http: //www. lancs. ac. uk/ug/jacksom 2/

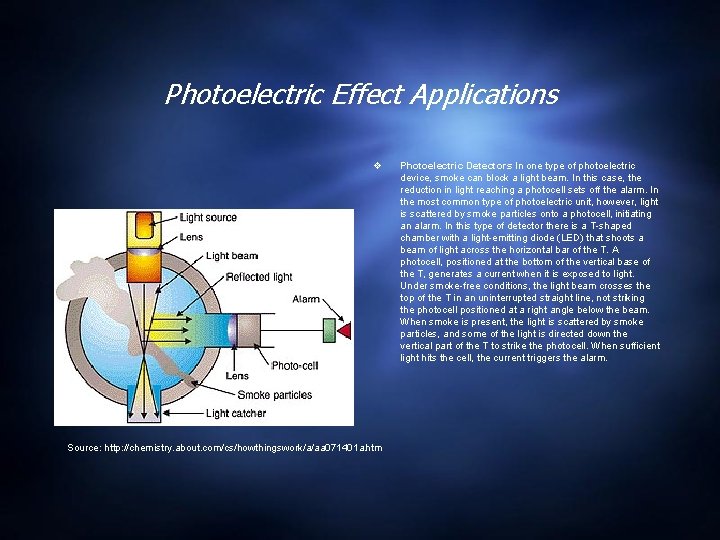
Photoelectric Effect Applications Source: http: //chemistry. about. com/cs/howthingswork/a/aa 071401 a. htm Photoelectric Detectors In one type of photoelectric device, smoke can block a light beam. In this case, the reduction in light reaching a photocell sets off the alarm. In the most common type of photoelectric unit, however, light is scattered by smoke particles onto a photocell, initiating an alarm. In this type of detector there is a T-shaped chamber with a light-emitting diode (LED) that shoots a beam of light across the horizontal bar of the T. A photocell, positioned at the bottom of the vertical base of the T, generates a current when it is exposed to light. Under smoke-free conditions, the light beam crosses the top of the T in an uninterrupted straight line, not striking the photocell positioned at a right angle below the beam. When smoke is present, the light is scattered by smoke particles, and some of the light is directed down the vertical part of the T to strike the photocell. When sufficient light hits the cell, the current triggers the alarm.

Photoelectric Smoke Detector Source: http: //www. bassburglaralarms. com/images_products/d 350 rpl_addressable_duct_smoke_detector_b 10685. jpg

Applications Solar panels are nothing more than a series of metallic plates that face the Sun and exploit the photoelectric effect. The light from the Sun will liberate electrons, which can be used to heat your home, run your lights, or, in sufficient enough quantities, power everything in your home. Source: www. futureenergy. org/ picsolarpannelsmatt. jpg

Work Cited Amar, Francois G. The Photoelectric Effect. 25 Sep 2003. Section of Chemistry 121 for fall 03. 11 May 2006 <http: //chemistry. umeche. maine. edu/~amar/fall 2003/photoelectric. html> Blawn, Jeramy R. and Colwell, Catharine H. Physics Lab: Photoelectric Effect. 10 Jun 2003. Mainland High School: Online Physics Labs. 11 May 20006 <http: //online. cctt. org/physicslab/content/Phy. APB/lessonnotes/dualnature/photoelectric. asp> Helmenstine, Anne Marie. Photoelectric & Ionization Smoke Detector. 25 Feb 2006. About. com. 11 May 2006 <http: //chemistry. about. com/cs/howthingswork/a/aa 071401 a. htm> Einstein, Albert. “Concerning an Heuristic Point of View Toward the Emission and Transformation of Light. ” American Journal Of Physics 5 May 1965: 137. Nave, Rod. Hyper. Physics. 19 Aug. 2000. Georgia State University. 06 May 2006 <http: //hyperphysics. phy-astr. gsu. edu/hbase/hframe. html>. Thornton T. , Stephen, and Rex, Andrew. Modern Physics for Scientists and Engineers. Canada : Thomson Brooks/Core, 2006 Photoelectric Effect. 24 Apr. 2006. Wikipedia Free Encyclopedia. 05 May 2006. <http: //en. wikipedia. org/wiki/Photoelectric_effect>.