Sampling Requirements for Mixture Analysis using Molecular Rotational



- Slides: 15

Sampling Requirements for Mixture Analysis using Molecular Rotational Resonance Spectroscopy Justin L. Neill, Matt T. Muckle, Aleksandr V. Mikhonin, Bright. Spec, Inc. 74 th International Meeting on Molecular Spectroscopy June 18, 2019 – Urbana, Illinois Talk TL 01 © 2019 Bright. Spec, Inc.

Introduction When we show analytical chemists rotational spectroscopy and discuss its many advantages, they always ask us… “How do you get to the sample get into the instrument? ” This seems like a very simple question, but another way to phrase it is…. “How do you ensure that what you see in your instrument can be related to what was in the original sample? ” This has motivated us to develop reliable, easy to use, and reproducible sampling interfaces for the introduction of both gas and condensed-phase samples into rotational spectroscopy instruments. 2 Pittcon 2019 – Philadelphia, PA SAS Special Symposium: March 19, 2019 © 2019 Bright. Spec, Inc. CP

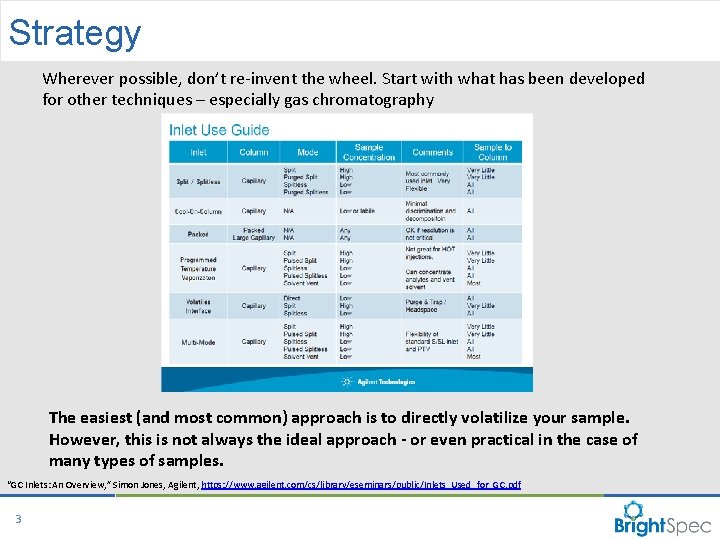
Strategy Wherever possible, don’t re-invent the wheel. Start with what has been developed for other techniques – especially gas chromatography The easiest (and most common) approach is to directly volatilize your sample. However, this is not always the ideal approach - or even practical in the case of many types of samples. “GC Inlets: An Overview, ” Simon Jones, Agilent, https: //www. agilent. com/cs/library/eseminars/public/Inlets_Used_for_GC. pdf 3 CP

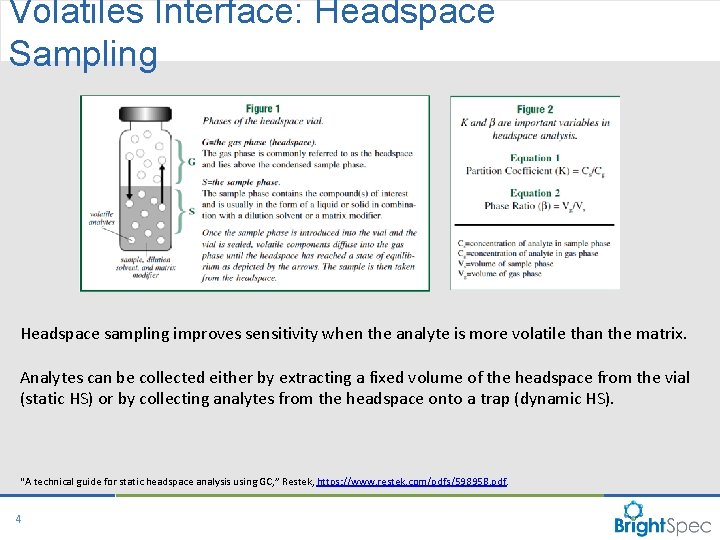
Volatiles Interface: Headspace Sampling Headspace sampling improves sensitivity when the analyte is more volatile than the matrix. Analytes can be collected either by extracting a fixed volume of the headspace from the vial (static HS) or by collecting analytes from the headspace onto a trap (dynamic HS). “A technical guide for static headspace analysis using GC, ” Restek, https: //www. restek. com/pdfs/59895 B. pdf. 4 CP

MRR Volatiles Interface Commercial autosampler can perform any sample mixing or dilution required, and inject sample into headspace vial Fully automated interface with autosampler, and transfer of vapor to chamber through a fixed-volume static headspace loop 5 CP

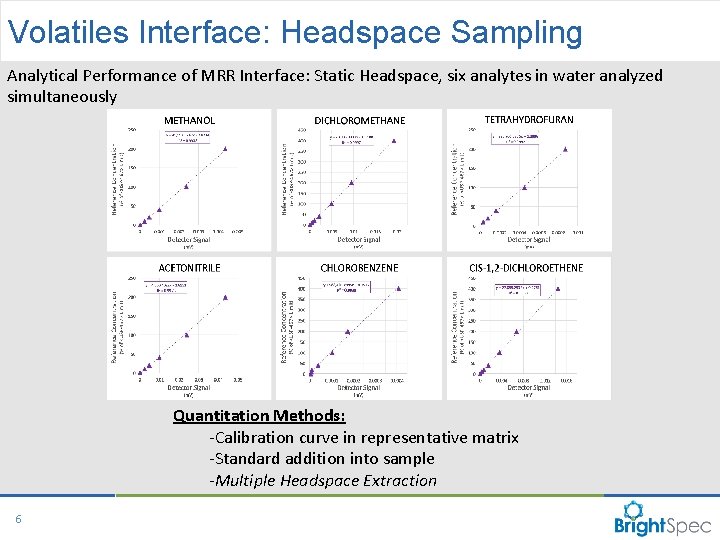
Volatiles Interface: Headspace Sampling Analytical Performance of MRR Interface: Static Headspace, six analytes in water analyzed simultaneously Quantitation Methods: -Calibration curve in representative matrix -Standard addition into sample -Multiple Headspace Extraction 6 CP

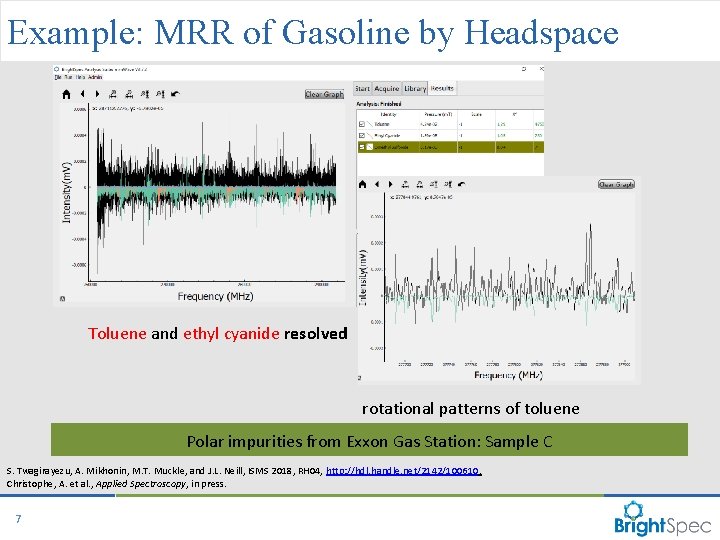
Example: MRR of Gasoline by Headspace Toluene and ethyl cyanide resolved rotational patterns of toluene Polar impurities from Exxon Gas Station: Sample C S. Twagirayezu, A. Mikhonin, M. T. Muckle, and J. L. Neill, ISMS 2018, RH 04, http: //hdl. handle. net/2142/100610. Christophe, A. et al. , Applied Spectroscopy, in press. 7 CP

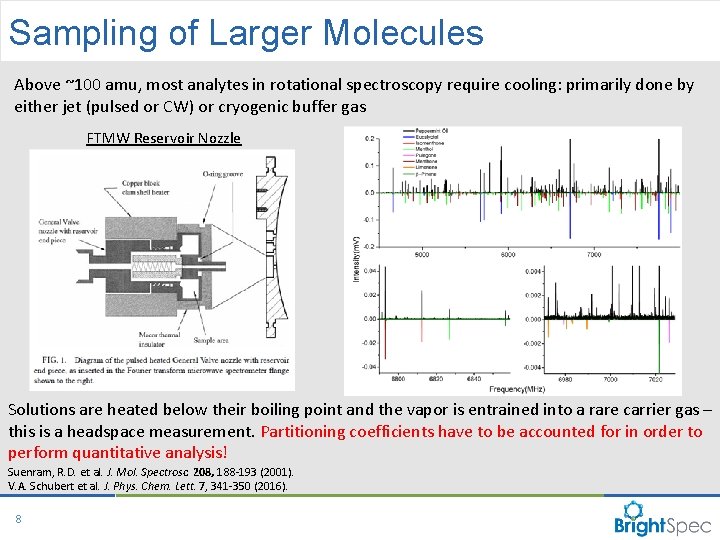
Sampling of Larger Molecules Above ~100 amu, most analytes in rotational spectroscopy require cooling: primarily done by either jet (pulsed or CW) or cryogenic buffer gas FTMW Reservoir Nozzle Solutions are heated below their boiling point and the vapor is entrained into a rare carrier gas – this is a headspace measurement. Partitioning coefficients have to be accounted for in order to perform quantitative analysis! Suenram, R. D. et al. J. Mol. Spectrosc. 208, 188 -193 (2001). V. A. Schubert et al. J. Phys. Chem. Lett. 7, 341 -350 (2016). 8 CP

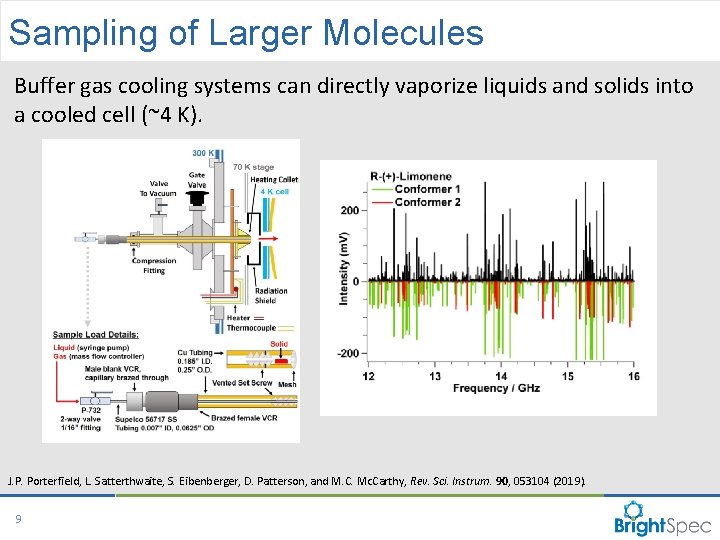
Sampling of Larger Molecules Buffer gas cooling systems can directly vaporize liquids and solids into a cooled cell (~4 K). J. P. Porterfield, L. Satterthwaite, S. Eibenberger, D. Patterson, and M. C. Mc. Carthy, Rev. Sci. Instrum. 90, 053104 (2019). 9 CP

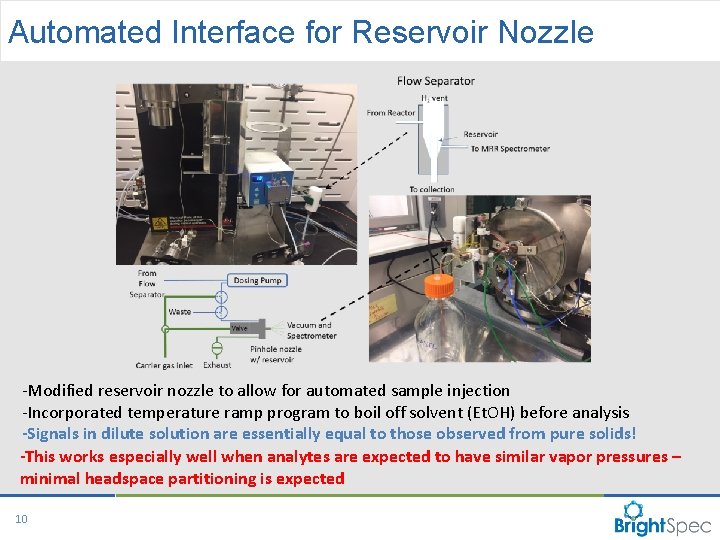
Automated Interface for Reservoir Nozzle -Modified reservoir nozzle to allow for automated sample injection -Incorporated temperature ramp program to boil off solvent (Et. OH) before analysis -Signals in dilute solution are essentially equal to those observed from pure solids! -This works especially well when analytes are expected to have similar vapor pressures – minimal headspace partitioning is expected 10 CP

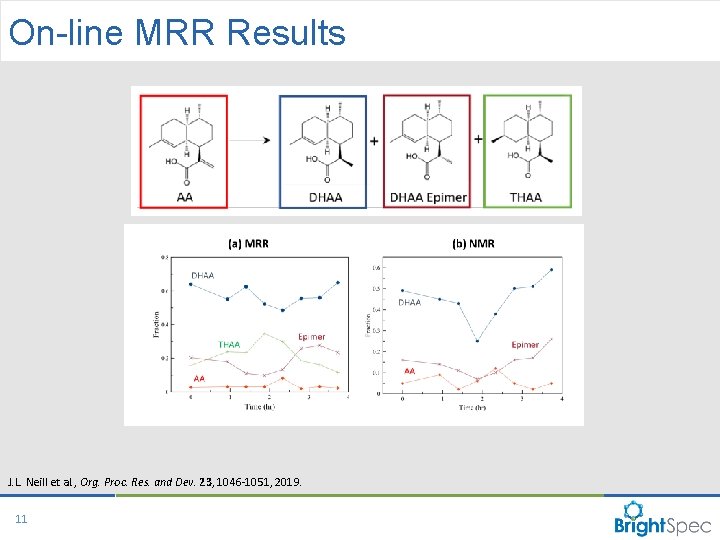
On-line MRR Results J. L. Neill et al. , Org. Proc. Res. and Dev. 23, 1046 -1051, 2019. 11 CP

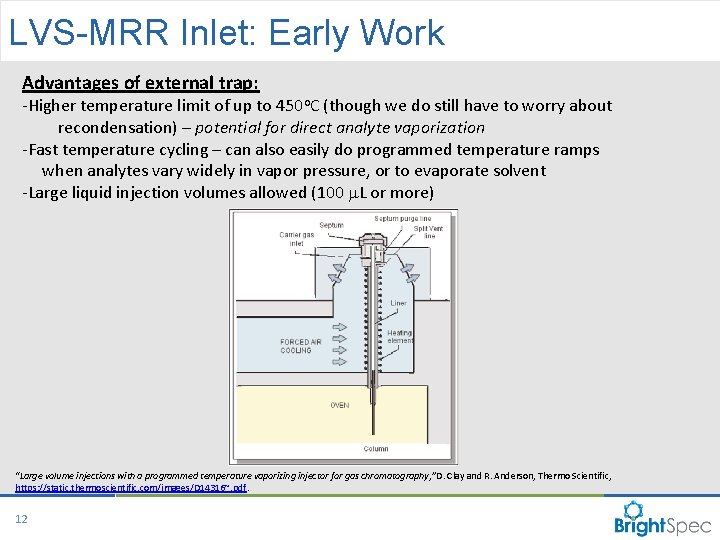
LVS-MRR Inlet: Early Work Advantages of external trap: -Higher temperature limit of up to 450 o. C (though we do still have to worry about recondensation) – potential for direct analyte vaporization -Fast temperature cycling – can also easily do programmed temperature ramps when analytes vary widely in vapor pressure, or to evaporate solvent -Large liquid injection volumes allowed (100 m. L or more) “Large volume injections with a programmed temperature vaporizing injector for gas chromatography, ” D. Clay and R. Anderson, Thermo Scientific, https: //static. thermoscientific. com/images/D 14316~. pdf. 12 CP

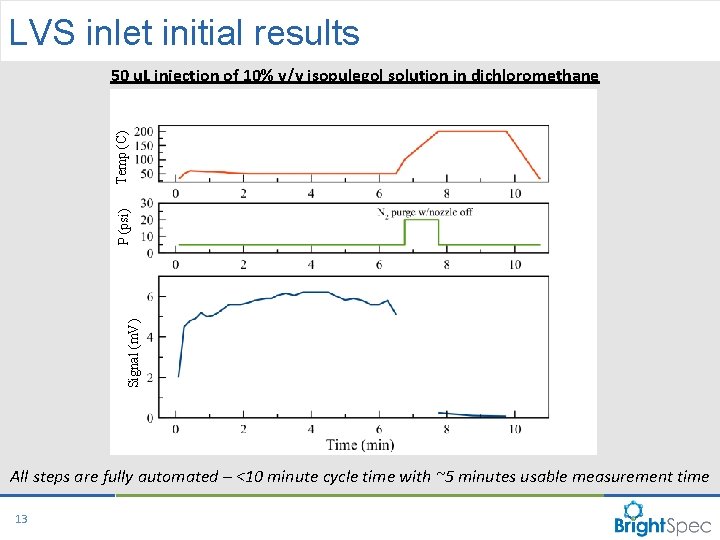
LVS inlet initial results Signal (m. V) P (psi) Temp (C) 50 u. L injection of 10% v/v isopulegol solution in dichloromethane All steps are fully automated – <10 minute cycle time with ~5 minutes usable measurement time 13 CP

Conclusions and Outlook Rotational spectroscopy has great analytical potential is in simplifying mixture analysis – motivating the development of easy-to-use sampling interfaces Progress has been made toward automated analysis both for volatiles sampling as well as initial steps toward automated large-molecule analysis Greatest end application may be in bringing challenging chemical resolution capabilities to automated processes – with potential for full automation Even for research studies, there are clear benefits to simplifying manual steps and increasing reproducibility between operators and instruments 14 CP

Acknowledgements Collaborators: - Virginia Commonwealth University: Frank Gupton, Yuan Yang, Tom Roper, Jo-Ann Jee - University of Virginia: Brooks Pate, Reilly Sonstrom - BASF: Reinhard Dötzer - Merck: Leo Joyce - Glaxo Smith Kline: Ted Chen, Yanan He Funding Support: Contract W 31 P 4 Q-16 -C-0068 SBIR #1556035 15 CP