Sampling microorganisms in water GwyAm Shin Department of



















- Slides: 36

Sampling microorganisms in water Gwy-Am Shin Department of Environmental and Occupational Health Sciences

The challenges • Different microbe types • Different water types • Low numbers of pathogens in natural waters

Different waterborne pathogens • • Viruses Bacteria Protozoa Helminths

Different type of waters • • Wastewater Surface water Ground water Source water Drinking water Recreational water Sea water Sediments and sludges

Low numbers of pathogens in water

Source • Environment – Mycobacterium avium complex (MAC) – Legionella pneumophila • Infected hosts – Humans – Animals

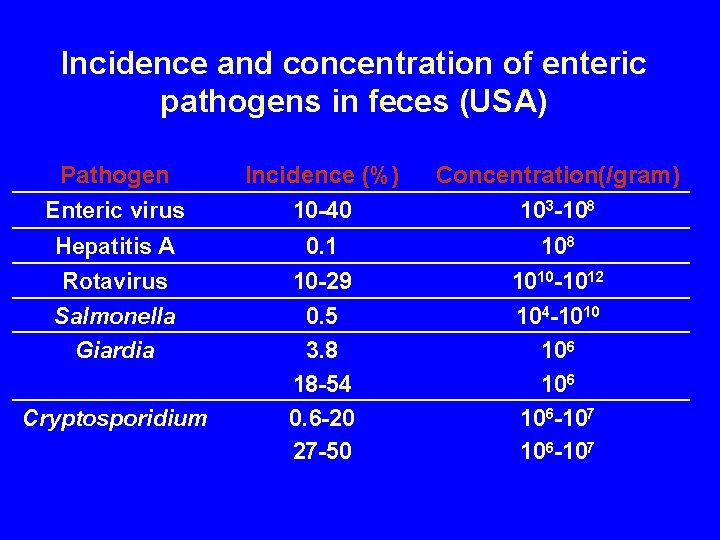
Incidence and concentration of enteric pathogens in feces (USA) Pathogen Enteric virus Hepatitis A Rotavirus Salmonella Giardia Cryptosporidium Incidence (%) 10 -40 0. 1 10 -29 0. 5 3. 8 18 -54 0. 6 -20 27 -50 Concentration(/gram) 103 -108 1010 -1012 104 -1010 106 106 -107

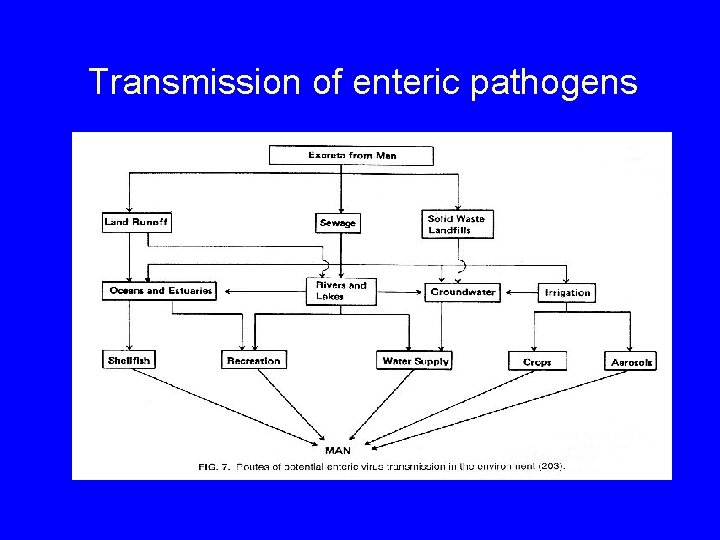
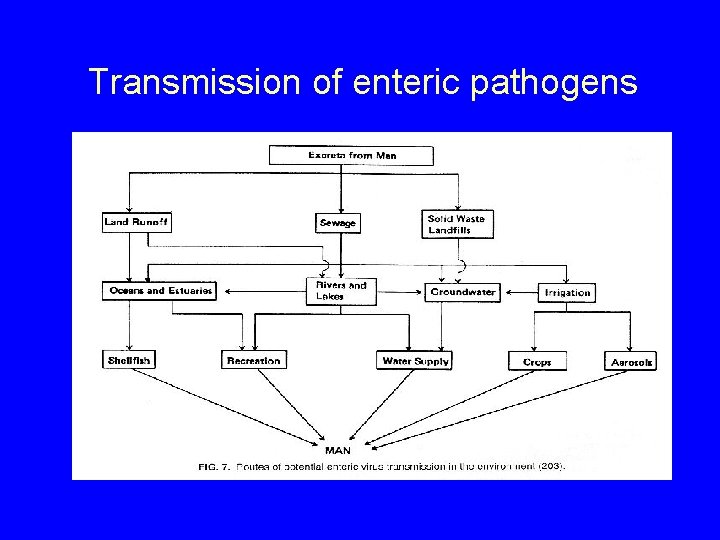
Transmission of enteric pathogens

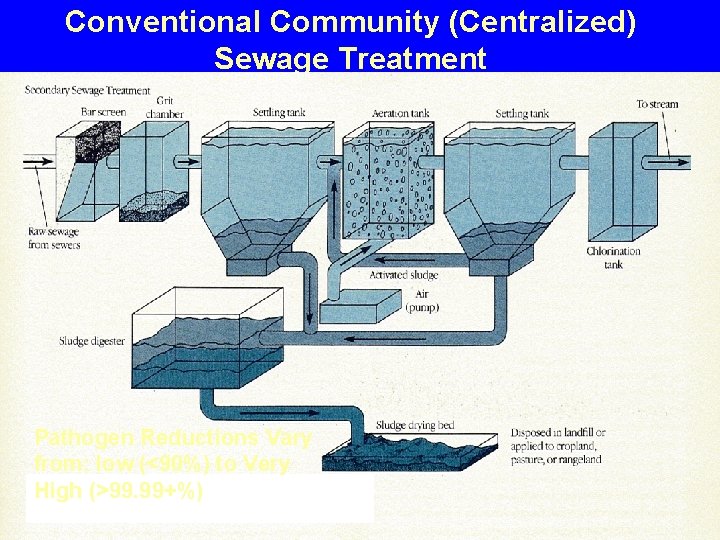
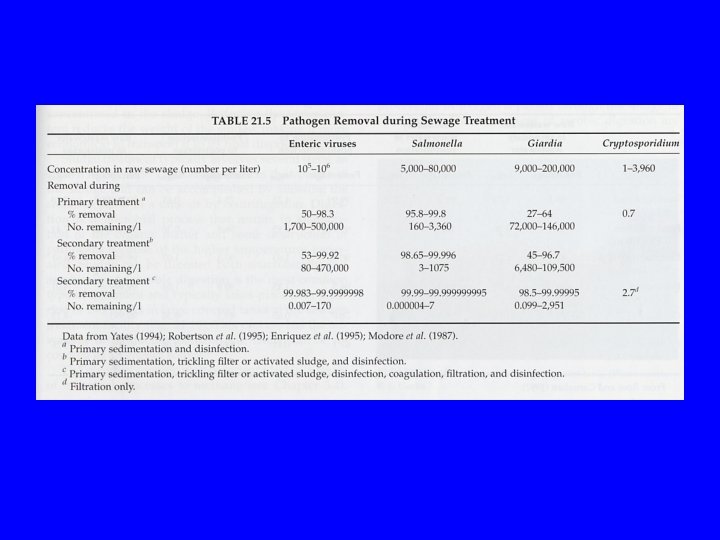
Conventional Community (Centralized) Sewage Treatment Pathogen Reductions Vary from: low (<90%) to Very High (>99. 99+%)


Transmission of enteric pathogens

Low number of microbes in natural waters • Need large volumes • Need to separate microbes from other materials

Steps in pathogen sampling in water • Concentration • Purification/Reconcentration • Analysis

Sampling enteric viruses in water

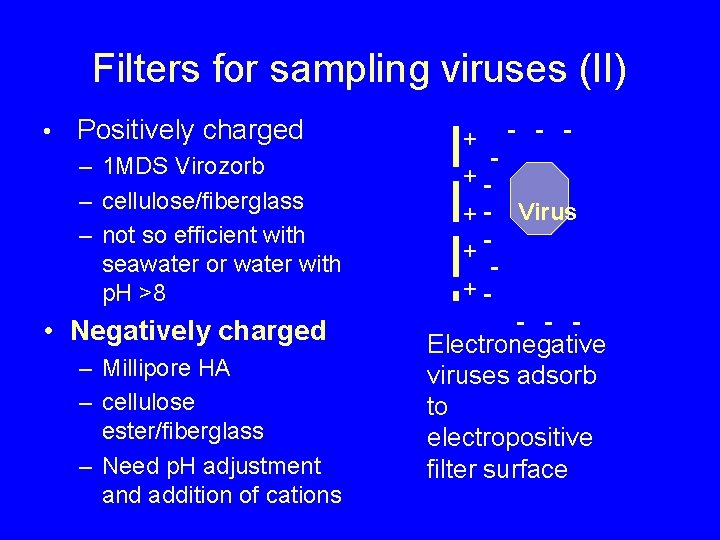
Filters for sampling viruses (I) • Adsorbent filters – pore size of filters (0. 2 -0. 45 µm) larger than viruses – viruses retained by adsorption – electrostatic and hydrophobic interactions • Positively charged and negatively charged filters

Filters for sampling viruses (II) • Positively charged – 1 MDS Virozorb – cellulose/fiberglass – not so efficient with seawater or water with p. H >8 • Negatively charged – Millipore HA – cellulose ester/fiberglass – Need p. H adjustment and addition of cations + - - - ++ - Virus ++- - - Electronegative viruses adsorb to electropositive filter surface

Different types of filters

Field sampling device for viruses

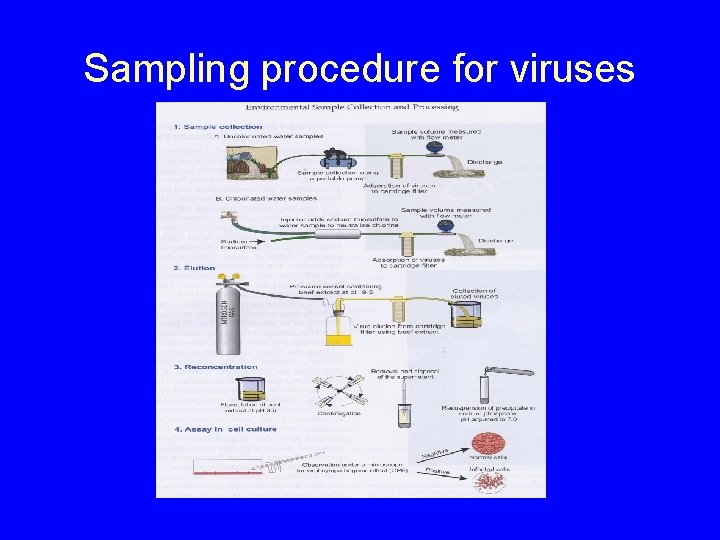
Sampling procedure for viruses

Elution from Adsorbent Filters • Choice of eluants – Beef extract – Amino acids – w/mild detergents • Considerations – Efficiency of elution – Compatibility with downstream assays – Volume – Contact time

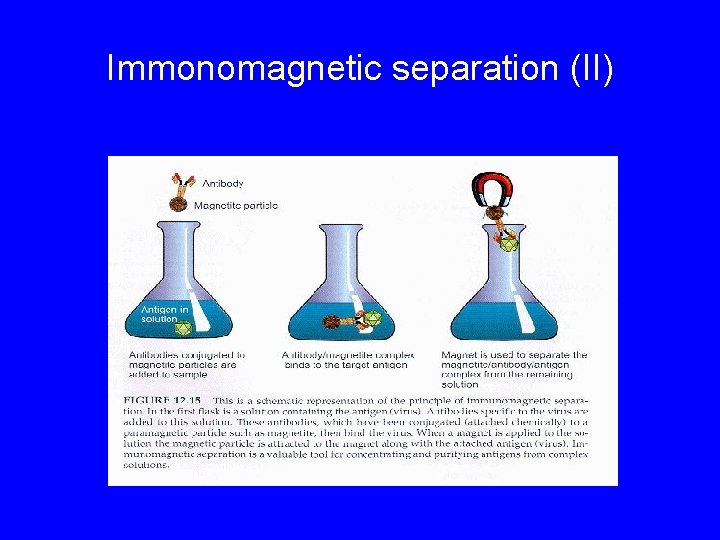
Reconcentration and Purification (Viruses) • Organic Flocculation • Adsorption to minerals (e. g. aluminum hydroxide, ferric hydroxide) • Hydroextraction (dialysis with Polyethylene Glycol (PEG)) • Spin Column Chromatography (antibodies covalently linked to gel particles) • IMS (Immunomagnetic separation) • Ligand capture

Immunomagnetic Separation (I) Y Bead Y Microbe Y Y Antibody

Immonomagnetic separation (II)

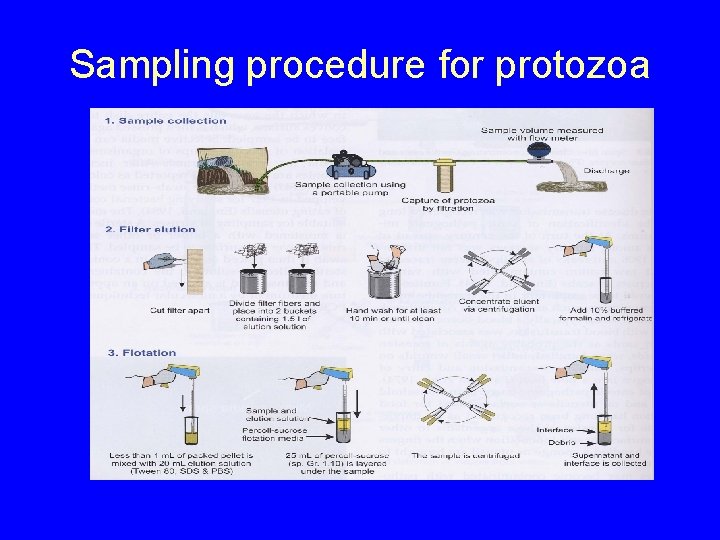
Sampling protozoan parasites in water

Filters for sampling protozoa in water • Size exclusion filters – 1 -several µm pore size – Protozoa retained by their sizes • Various formats – Cartridge, capsule, and disk filters

Different types of filters

Sampling procedure for protozoa

Elution from size exclusion filters • Choice of eluants – PBS with Tween 80 and SDS (sodium dodecyl sulfate) – Tris buffer with laureth-12, EDTA, and antiform A

Reconcentration and Purification (Protozoa) • Flocculation with calcium carbonate • Membrane filtration • Ultrafiltration • IMS (Immunomagnetic separation) • Floatation/ Buoyant density gradient centrifugation

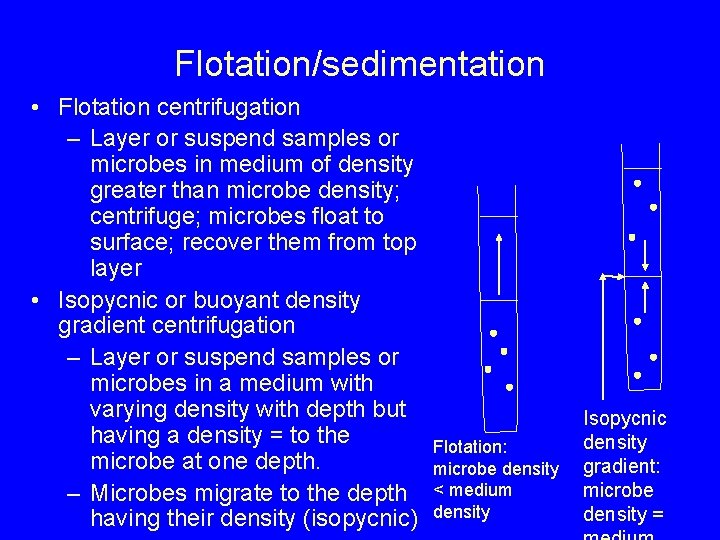
Flotation/sedimentation • Flotation centrifugation – Layer or suspend samples or microbes in medium of density greater than microbe density; centrifuge; microbes float to surface; recover them from top layer • Isopycnic or buoyant density gradient centrifugation – Layer or suspend samples or microbes in a medium with varying density with depth but having a density = to the microbe at one depth. – Microbes migrate to the depth having their density (isopycnic) Flotation: microbe density < medium density Isopycnic density gradient: microbe density =

Sampling and analysis for bacteria in water

Indicator bacteria • • Total coliforms Fecal coliforms E. coli Enterococcus

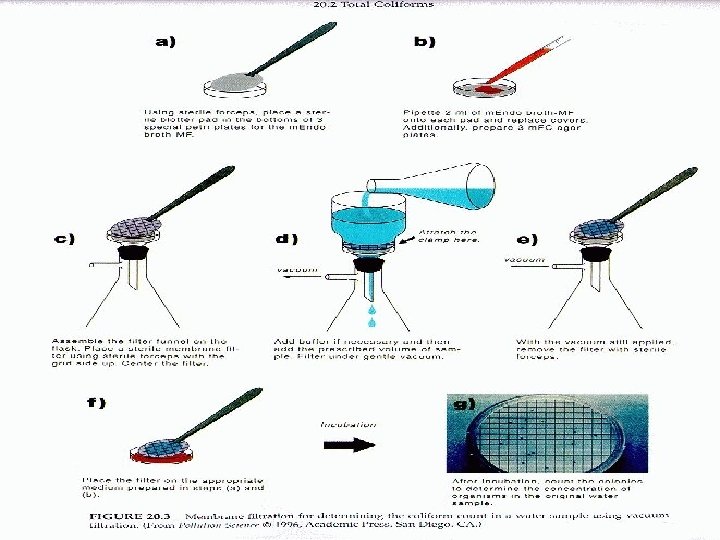
Membrane filtration technique • Waters with relatively high bacteria numbers • Filtration (0. 45 µm nitrocellulose) • Growth on a selective solid medium


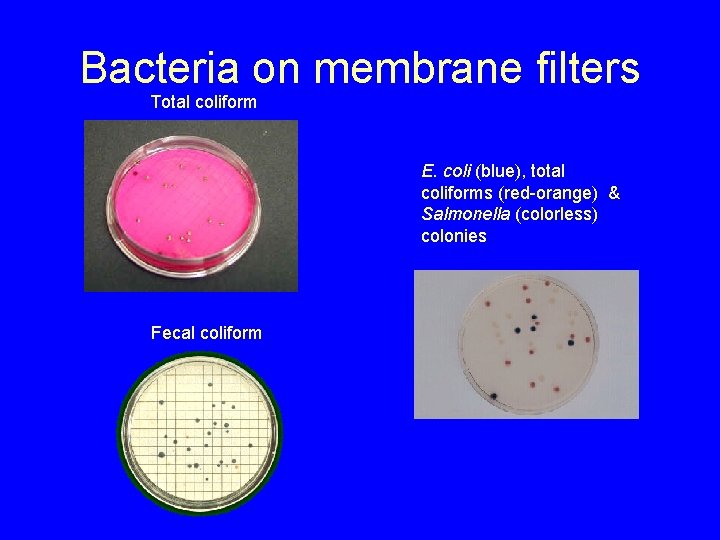
Bacteria on membrane filters Total coliform E. coli (blue), total coliforms (red-orange) & Salmonella (colorless) colonies Fecal coliform

Conclusions • Sampling methods are lagging behind detection methods – There is a need to focus on the reliability and sensitivity of concentration methods – Negative results don’t necessarily mean target not there • Difficulties with a single platform for any one media because of wide range of organisms and environmental conditions
 Water and water and water water
Water and water and water water Intrinsic factors affecting microbial growth
Intrinsic factors affecting microbial growth Probability vs non probability sampling
Probability vs non probability sampling Difference between stratified and cluster sampling
Difference between stratified and cluster sampling Probability sampling
Probability sampling Yuli fajar susetyo
Yuli fajar susetyo Cluster random sampling vs stratified
Cluster random sampling vs stratified Quota sampling vs random sampling
Quota sampling vs random sampling Natural sampling vs flat top sampling
Natural sampling vs flat top sampling Tung shin hospital charges
Tung shin hospital charges Dr elizabeth shin
Dr elizabeth shin Shin kubota immortal jellyfish
Shin kubota immortal jellyfish Parts of the head
Parts of the head Symanti
Symanti Osi shin guards
Osi shin guards How to wrap a thumb with athletic tape
How to wrap a thumb with athletic tape Nejime floral design
Nejime floral design Rikkwa
Rikkwa Shin inouye
Shin inouye Frecombo
Frecombo Main characters in the westing game
Main characters in the westing game Shin sobue
Shin sobue Kim shin jo
Kim shin jo Learning objectives of microorganisms
Learning objectives of microorganisms Microbes importance
Microbes importance Harmful microorganisms
Harmful microorganisms Harmful microorganisms
Harmful microorganisms The five i's of studying microorganisms
The five i's of studying microorganisms Flora ir fauna
Flora ir fauna Limitations of light microscope
Limitations of light microscope Microorganisms meaning
Microorganisms meaning Microorganisms 5th grade
Microorganisms 5th grade Organism
Organism Fermentation in microorganisms
Fermentation in microorganisms Microorganism wanted poster
Microorganism wanted poster What is microbiology
What is microbiology Classification of microorganisms
Classification of microorganisms