SAMPLE ANALYSIS AT MARS SAM Introduction The Sample




















- Slides: 37

SAMPLE ANALYSIS AT MARS (SAM)

Introduction The Sample Analysis at Mars (SAM) suite of instruments is designed to explore the past or present habitability of Mars by exploring carbon chemistry relevant to life. Sample Analysis at Mars (SAM) is a suite of instruments on the Mars Science Laboratory Curiosity Rover. The SAM instrument suite will analyze organics and gases from both atmospheric and solid samples. It will conduct a sensitive search for organic compounds and measure the isotopic composition of carbonaceous material.

Goal 1 : Survey carbon compound sources and evaluate their possible mechanism of formation and destruction. Goal 2 : Search for organic compounds of biotic and prebiotic importance including methane. Goal 3 : Reveal the chemical and isotopic state of elements (i. e. N, H, O, S, C and others) are important for life as we know it.

Goal 4 : Evaluate the habitability of Mars by studying its atmospheric chemistry and the composition of trace species that are evidence of interactions between the atmosphere and soil. Goal 5 : Understand atmospheric and climatic evolution through measurements of noble gas and light element isotopes.

THE GOALS OF THE SAM INVESTIGATION ARE TO INVESTIGATE THREE QUESTIONS: 1. What does the inventory of carbon compounds, or lack there of, near the surface of Mars reveal about its potential habitability? 2. What are the chemical and isotopic states of the lighter elements in the rocks, soils, and atmosphere and what do these reveal about potential habitability? 3. How were past environmental con ditions different from today?

WHAT DOES SAM DO…? ? It can detect carbon compounds in Mars rock and soil samples, if they exist, and can tell us what compounds they are. It can measure isotopic ratios of light elements. The compounds it finds can tell us about the environmental conditions that prevailed when they form. The Sample Analysis at Mars, or SAM, is about the size of a microwave oven.

SAM :

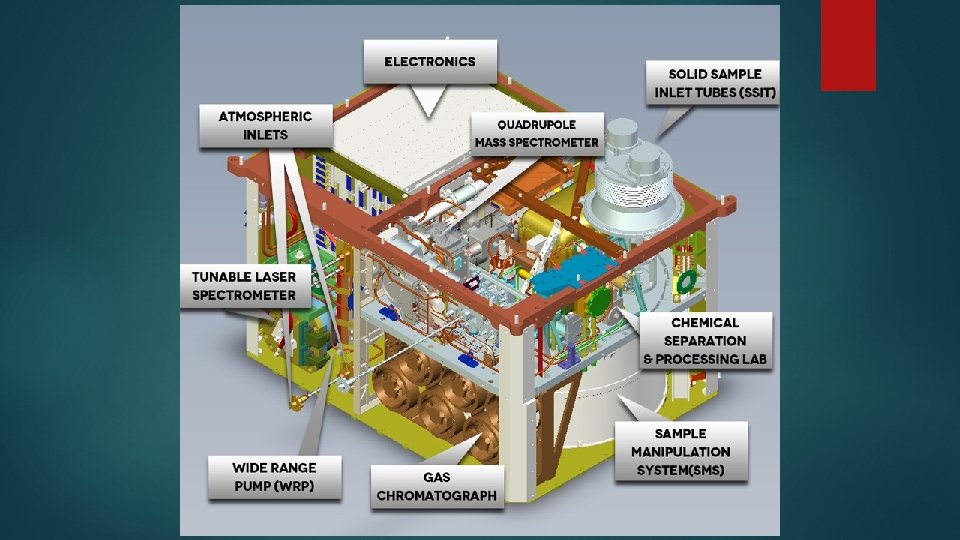
Requirements § § § Atmospheric inlets Quadrupole Mass Spectrometer Solid Sample Inlet Tubes(SSIT) Gas chromatograph Sample Manipulation System(SMS) Tunable Laser Spectrometer(TLS)


This is sam being installed inside rover, which had been rotated upside down for instrument installation. This is a really big instrument, which is weighing in at 40 Kilograms.

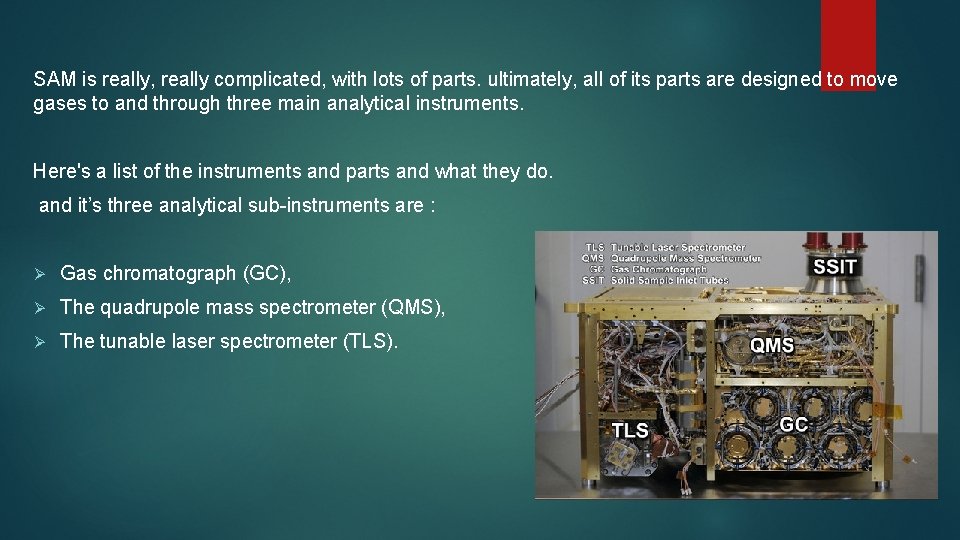
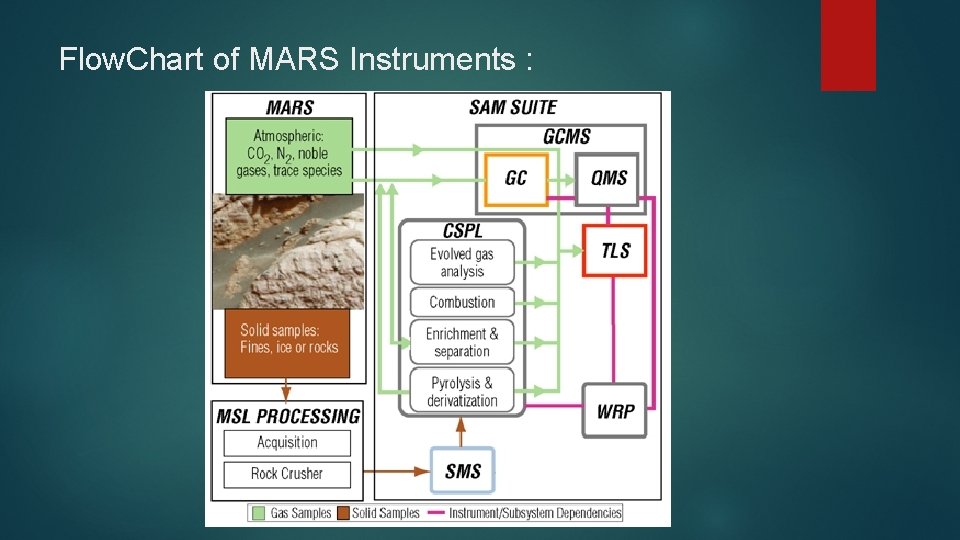
SAM is really, really complicated, with lots of parts. ultimately, all of its parts are designed to move gases to and through three main analytical instruments. Here's a list of the instruments and parts and what they do. and it’s three analytical sub-instruments are : Ø Gas chromatograph (GC), Ø The quadrupole mass spectrometer (QMS), Ø The tunable laser spectrometer (TLS).

GAS CHROMATOGRAPH (GC): Ø The gas chromatograph allows curiosity to separate a mixture of gases, which improves the mass spectrometer's ability to identify them. Ø The separation is done in chromatographic columns (cc, metallic capillary tubes). Ø The choice to use 6 columns is due to the requirement to analyze simultaneously a wide variety of organic and inorganic compounds. Ø Gas chromatography helps a mass spectrometer distinguish among different organic compounds that have the same molecular weights.

Ø There actually six different gas chromatograph "columns“. Ø Each of the six columns is a tube 30 meters long but only a quarter of a millimeter in diameter. Ø The tubes are wound into coils to pack them inside the instrument, so each of the "columns“ actually looks like a disk, from the outside.

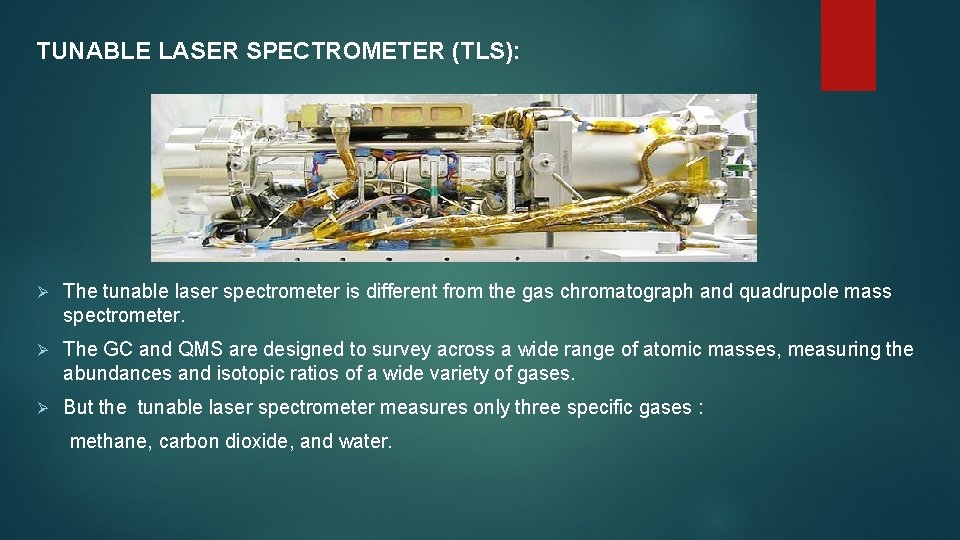
TUNABLE LASER SPECTROMETER (TLS): Ø The tunable laser spectrometer is different from the gas chromatograph and quadrupole mass spectrometer. Ø The GC and QMS are designed to survey across a wide range of atomic masses, measuring the abundances and isotopic ratios of a wide variety of gases. Ø But the tunable laser spectrometer measures only three specific gases : methane, carbon dioxide, and water.


Ø It can make very precise measurements of the isotopic ratios of hydrogen, carbon, and oxygen that they contain by looking at specific-wavelength emission lines of the different isotopes of those three elements. Ø The tunable laser spectrometer doesn't operate continuously, unlike the QMS. Ø SAM waits until the TLS chamber is filled to some desired pressure, and then performs a tls reading. then it usually will pump out some of the gas and perform another reading at a lower pressure. Ø It usually repeats this a few times. reading at multiple different pressures makes sure that there will be at least one reading for each gas at which the detector is not saturated. Ø TLS will measure the 13 c/12 c isotope ratios in both ch 4 and co 2.

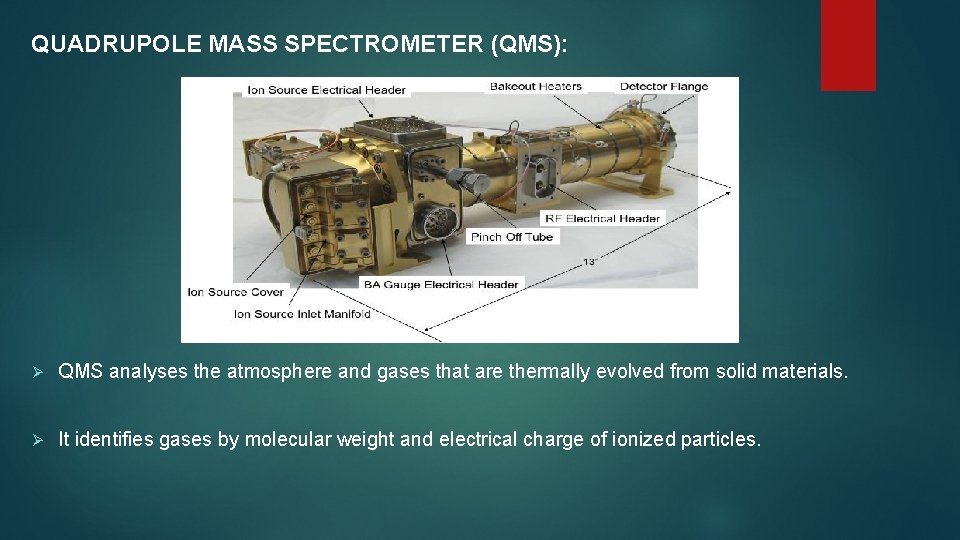
QUADRUPOLE MASS SPECTROMETER (QMS): Ø QMS analyses the atmosphere and gases that are thermally evolved from solid materials. Ø It identifies gases by molecular weight and electrical charge of ionized particles.

Ø Electrons are fired at the molecules breaking them into fragments and sorting those by establishing an electric field. Ø QMS provides spectra that are used to identify the molecules. QMS can operate in static or dynamic mode.


EXPERIMENTS PERFORMED ON ATMOSPHERIC SAMPLES : Ø To perform any atmospheric analysis, SAM begins by activating its pumps and performing background readings from QMS and TLS. Ø Here the SAM heats the manifolds and tubes that it will use to transfer the gas from outside the rover to the instruments. (a typical temperature is 135°c. ) once the instrument is at temperature, SAM opens a valve to take in air. THERE ARE FOUR EXPERIMENTS PERFORMED ON ATMOSPHERIC SAMPLES THEY ARE : i. DIRECT ANALYSIS OF ATMOSPHERIC GAS ii. NOBLE GAS ANALYSIS iii. METHANE ANALYSIS iv. ATMOSPHERIC ENRICHMENT .

I. DIRECT ANALYSIS OF ATMOSPHERIC GAS : Ø This will be the most frequently performed atmospheric sample experiment, involving mass spectrometry and laser spectrometry measurements of the atmosphere. Ø SAM performs a QMS measurement first, then it uses the TLS. The TLS measurement is performed several times with the pumps establishing different pressures inside the instrument.

II. NOBLE GAS ANALYSIS : Ø Argon and neon are the most abundant noble gases in mars' atmosphere, so they comprise the bulk of the remaining gas. Ø This remaining gas can be directed to the QMS for isotopic analysis. or it can be sent to another manifold, where a thermoelectric cooler traps the heavier noble gases xenon and krypton. Ø After SAM pumps out the (mostly argon and neon) remaining gas, it warms the cold trap to release the xenon and krypton to be pumped to the QMS, again for isotopic analysis.

III. METHANE ANALYSIS : Ø In a procedure similar to the noble gas analysis, SAM uses the getters and scrubbers to remove carbon dioxide and nitrogen from the air. Ø The remaining gas (which is mostly argon, but which will contain some of the methane) is directed to the TLS. Ø SAM repeats this procedure multiple times to raise the density of methane gas in the TLS-- if it's there at all.

IV. ATMOSPHERIC ENRICHMENT : Ø Sort of the opposite of the methane analysis, SAM directs atmospheric gas over the scrubber, then pumps out the remainder and releases the trapped gases. Ø The scrubber's main purpose is to take up carbon dioxide and water, but it also traps hydrocarbons heavier than methane as well as other trace gases. Ø So, whatever has been taken up by the scrubber is released either to the TLS -- which can then perform an isotopic analysis of the water, which can later be released to the QMS or GC.

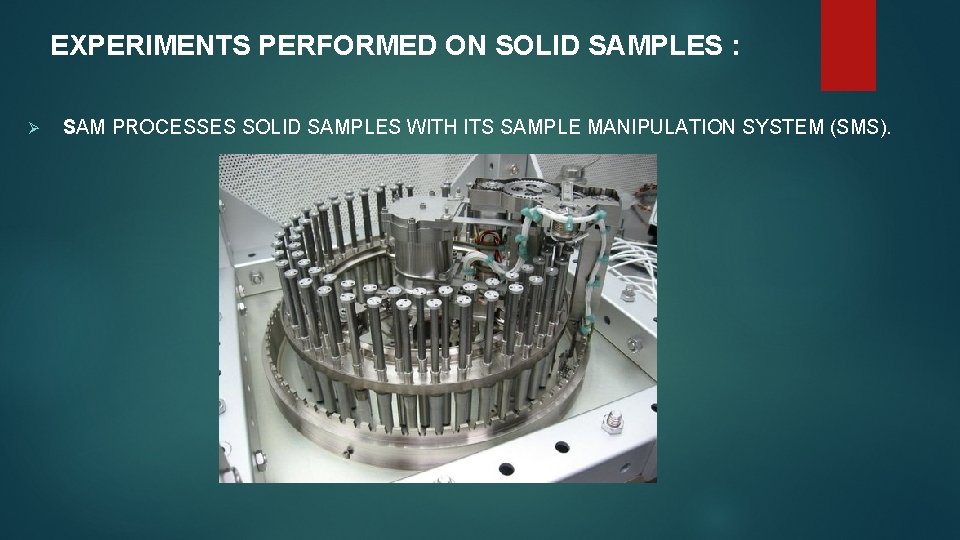
EXPERIMENTS PERFORMED ON SOLID SAMPLES : Ø SAM PROCESSES SOLID SAMPLES WITH ITS SAMPLE MANIPULATION SYSTEM (SMS).

Ø The heart of the sample manipulation system is a carousel containing 74 sample cups in two concentric rings. Ø There are three main kinds of cups: 59 quartz cups, 9 wet chemistry cups, and 6 calibration cups. Ø The carousel can deliver individual cups to one of two ovens by rotating to place the chosen cup underneath the oven. Ø It shoves upward with force powerful enough to create a tight seal between the top of the sample cup and the roof of the oven.

There are ways that the evolved gas analysis can be improved. at certain times, some of the evolved gas can be directed into the TLS , where it will be held for later analysis of water, carbon dioxide, and methane abundance and isotopes. 1. Gas chromatograph mass spectrometer analysis. 2. Tunable laser spectrometer analysis. 3. Solid sample combustion. 4. Wet chemical analysis.

1. GAS CHROMATOGRAPH MASS SPECTROMETER ANALYSIS: I. After an evolved gas analysis, SAM will usually go on to perform gas chromatography and mass spectroscopy of the stuff that was caught in the hydrocarbon trap. Ii. SAM heats its hydrocarbon trap, which releases the stuff that stuck to it. Iii. It sends the released hydrocarbon gases through one of the six gas chromatograph columns. Iv. As the gas exits the column, it's measured with thermal conductivity detector and then with the mass spectrometer.

2. TUNABLE LASER SPECTROMETER ANALYSIS : i. Once the gas chromatograph analysis is done, SAM will usually go on to use the tunable laser spectrometer to look at abundances of and isotopes in water, methane, and carbon dioxide. ii. The whole sequence of evolved gas analysis - gas chromatograph mass spectroscopy - tunable laser spectrometry takes 4 to 6 hours to complete. iii. If there's not enough power available to do all three analyses in one sol, the activities can be spaced out over multiple sols.

3. SOLID SAMPLE COMBUSTION : i. After evolved gas analysis, there will almost always be a filtration left inside the sample cup. ii. Even some carbon compounds may not become volatile even at the highest temperatures SAM's ovens can achieve. iii. SAM contains a reservoir of oxygen. with oxygen puffed into the oven, heating the cup above 750°c for some period turns refractory carbon compounds into (primarily) carbon dioxide. iv. By sending this gas to the TLS, SAM can determine the isotopic ratio of ordinary carbon-12 to heavy carbon-13 in the material. v. SAM can actually reuse the sample cup for another, later analysis, and the filtration shouldn't contaminate the results.

4. WET CHEMICAL ANALYSIS : i. Some of the most interesting carbon compounds that curiosity might find are ones that SAM can't pick up through any of the above methods. ii. These are large, astrobiologically interesting compounds like amino acids and carboxylic acids. and they aren't volatile at low temperatures. iii. They rapidly decompose to smaller compounds at higher temperatures, so they're essentially invisible.

Flow. Chart of MARS Instruments :

WHAT WILL THE SAM DATA SET BE…? SAM is required to obtain specific data sets to specific performance levels. Ø Inventory volatile organic compounds in rocks and soils, measuring anything that's more abundant than a few parts per billion (by mass). Ø Inventory certain specific astrobiologically interesting molecules including amino acids, amines, and carboxylic acids. Ø Measure the carbon-13/carbon-12 ratio in refractory carbon compounds. this is where the combustion analysis comes in. SAM is required to measure abundances of such refractory compounds to the part-per-million level. Ø Measure the distribution of oxidation states of organic compounds. this gets at the environmental conditions that prevailed when they formed.

Ø Inventory and temperature-profile volatile inorganic compounds in rocks and soils. this will get at abundances of certain minerals present in the rocks, like carbonates, sulfates, and clays, to the part-per-million level. Ø Measure the abundance and carbon-13/carbon-12 ratio of methane. Ø Measure how atmospheric gas concentrations vary over the course of a sol and over the course of mars' seasons. Ø Measure the relative abundances and isotopic ratios of the noble gases argon, neon, xenon, and krypton. And there are specific precision levels required for all these abundances and ratios

Constraints Ø It consists of instruments developed by the nasa goddard space flight center, the laboratoire inter-universitaire des systèmes atmosphériques (lisa). Ø It will measure the abundances of other light elements, such as hydrogen, oxygen, and nitrogen, associated with life. Ø SAM's solid sample inlet tubes (SSIT), visible at top right, are the routes by which curiosity's robotic arm will deliver samples of soil and powdered rock for analysis. Ø Other major components of SAM include the sample manipulation system and the gas processing system.

Ø SAM is a power-hungray instruments. This is a power for a full GC/QMS/TLS sequence lasting nearly six hours. Ø The sample manipulation system (SMS) for transporting powder delivered from the MSL drill to a SAM inlet and into one of 74 sample cups. Ø The SMS then moves the sample to the SAM oven to release gases by heating to up to 1000 o. C. Ø SAM are more than 600 meters (more than 650 yards) of wiring, 52 microvalves, a softdrink-can-size pump that rotates 100, 000 times per minute, and many other components.

COMMANDS § SAM_ON § SAM_OFF § SAM_OPEN § SAM_CLOSE § SAM_CLEAN § SAM_RELEASE