Salts and Electrolysis Lab Ionic compounds Made of

Salts and Electrolysis Lab

Ionic compounds • Made of • positive ions = cations (metal or polyatomic ion) -AND • negative ions = anions (nonmetal or polyatomic ion) • Salts = ionic compounds • Greater the electronegativity difference the more ionic – See table S for eletronegativities – Difference in electronegativities >1. 7 = ionic

Hydrolysis = salts dissociate in water cations and anions (aqueous solution) • • • Ex: Na. Cl + H 2 O Na+(aq) + Cl-(aq) Cations = Na+ Anions = Cl. Note: what happens to the water – some salts react with water solutions of acids, bases, ions and salts.

1) What happens when you dissolve salts in water? • They form ions in solution

Gypsum Springs, Axel Heiburg Glacial melt flows through Gypsum Hill Gypsum Springs

Ca. SO 4 = gypsum 2) What ions form when gypsum salts dissolve in water? Ca. SO 4(s) + H 2 O ___ Be sure to write the proper oxidation # that goes with each • Ca+2(aq) = cations • SO 4 -2(aq) = anions

Aqueous solutions = Electrolytes • Electrolytes = ionic solutions that conduct electricity • Add salt to water salt ions • Salt ions increase conductivity of water • How can we test this? ? ?
Construct a Voltaic Cell (testing electrolytes)

Pre-lab Questions • Using Table J which metal is more active Mg or Cu? • Mg and Cu act as electrodes (places where electrons move to and from. ) Metals like to lose electrons so electrons flow from more active metals to less active ones. Which way will electrons flow if we use Mg and Cu electrodes? • From Mg to Cu • Which one will act as the anode? • Mg
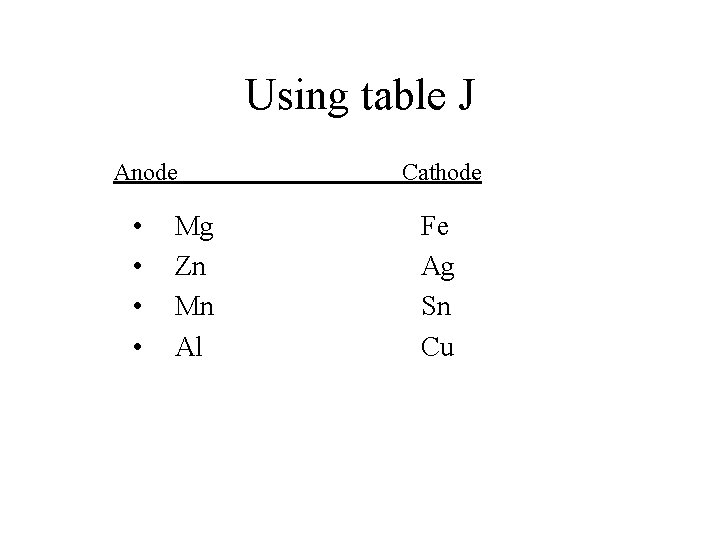
Using table J Anode • • Mg Zn Mn Al Cathode Fe Ag Sn Cu
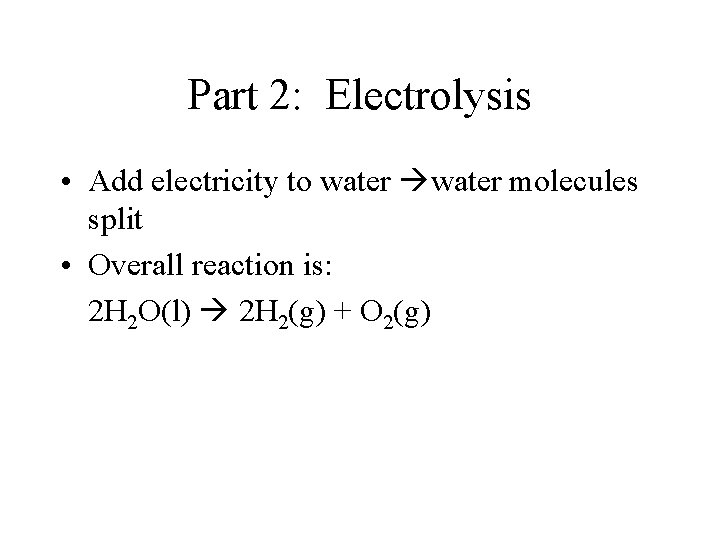
Part 2: Electrolysis • Add electricity to water molecules split • Overall reaction is: 2 H 2 O(l) 2 H 2(g) + O 2(g)
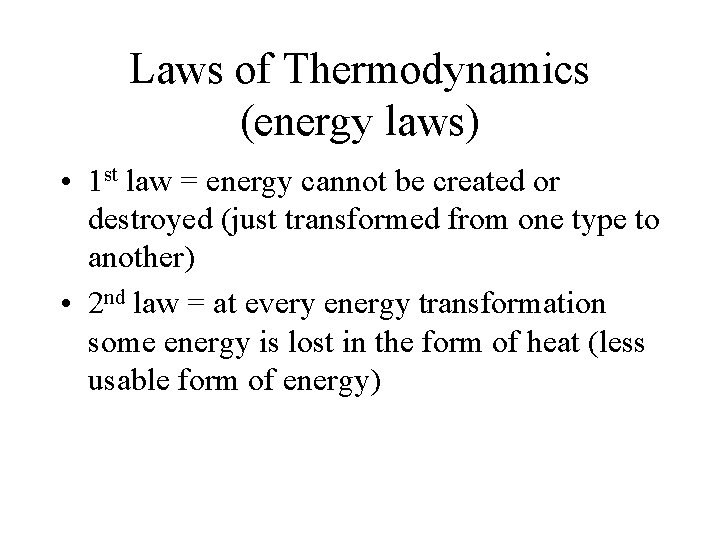
Laws of Thermodynamics (energy laws) • 1 st law = energy cannot be created or destroyed (just transformed from one type to another) • 2 nd law = at every energy transformation some energy is lost in the form of heat (less usable form of energy)

Set up your electrolysis apparatus
Follow lab procedures for comparing electrolytes
- Slides: 14