Salt Hydrolysis Salts Ionic compound made up of

















- Slides: 21

Salt Hydrolysis

Salts Ionic compound made up of CATION and ANION Has acidic and basic properties Based on ions produced when salts dissociate No acid/base properties—group I/II cations (ex. Na+, Li+, K+, Ca+2) No basic properties—conjugate bases from monoprotic acids (ex. Cl-, Br-, NO 3 -) Ex. Na. Cl, Ca. Br 2

Salt Hydrolysis Acid-base reactions between ions and water What do we mean by acidic, basic, or neutral salts? Think about how salt is formed……

1. Salt Formation from Strong Base and Weak Acid Salt forms a BASIC solution. Conjugate base ion reacts with water to give hydroxide (OH-) ions. Ex. Potassium fluoride (KF) KF K+ + F F- + H 2 O HF + OH-

2. Salt Formation from a Strong Acid and Weak Base Salt forms an ACIDIC solution Conjugate acid reactions with water to give hydronium ion (H 3 O+) Ex. Ammonium nitrate (NH 4 NO 3)

3. Salt Formation from Strong Acid and Strong Base Salt forms a NEUTRAL solution Conjugate base resulting from salt dissociation is weak Ex. Sodium chloride (Na. Cl)

Salt dissociation into ions that form conjugates of weak acids or bases hydrolyze in a predictable manner. (IE salts coming from either a strong base or a strong acid)

Calculations involving salt hydrolysis 1) Write the dissociation of the salt. 2) Determine what type of salt (acidic or basic) is present. 3) Write the reaction of the salt with water 4) Solve using an ICE chart

Example 1: Calculate the concentration of HOAc, OAc- and OH- at equilibrium in a 0. 10 M Na. OAc solution (Ka for HOAc = 1. 8 x 10 -5).

Example 2: Calculate the p. H of a 0. 052 M NH 4 Cl solution.

Example 3: What is the molarity of a CH 3 COONa solution that has a p. H = 9. 10?

Types of Acid-Base Reactions 1) Strong Acid/Strong Base 2) Weak Acid/Strong Base 3) Strong Acid/Weak Base 4) Weak Base/Weak Acid

1) Strong Acid/Strong Base Reaction goes to completion, K = 1 x 10 -14, p. H = 7 H 3 O+ + OH- H 2 O(l) + H 2 O(l) Always this net ionic equation H+ and OH- form H 2 O Commonly referred to as “neutralization reactions”

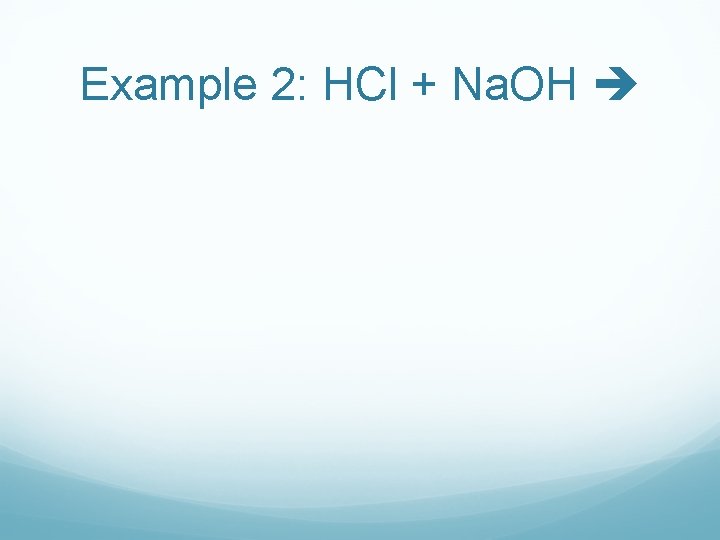
Example 2: HCl + Na. OH

2) Weak Acid/Strong Base Produces H 2 O and the conjugate base from weak acid Reaction goes MOSTLY to completion Solution’s p. H is more dependent on CONJUGATE Base (A-)’s reaction with water Find equilibrium constant (K) by combining known dissociation reactions to get the correct overall equation and solve for K

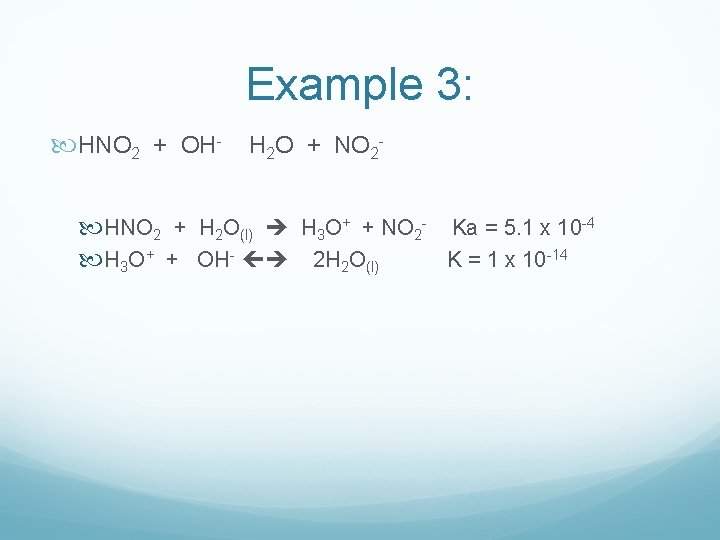
Example 3: HNO 2 + OH- H 2 O + NO 2 HNO 2 + H 2 O(l) H 3 O+ + NO 2 - Ka = 5. 1 x 10 -4 H 3 O+ + OH- 2 H 2 O(l) K = 1 x 10 -14

3. Strong Acid/Weak Base Weak bases tend to contain nitrogen-based compounds such as NH 3 Weak bases accept protons from acid Combine known dissociation reactions to get the correct overall equation and solve for K

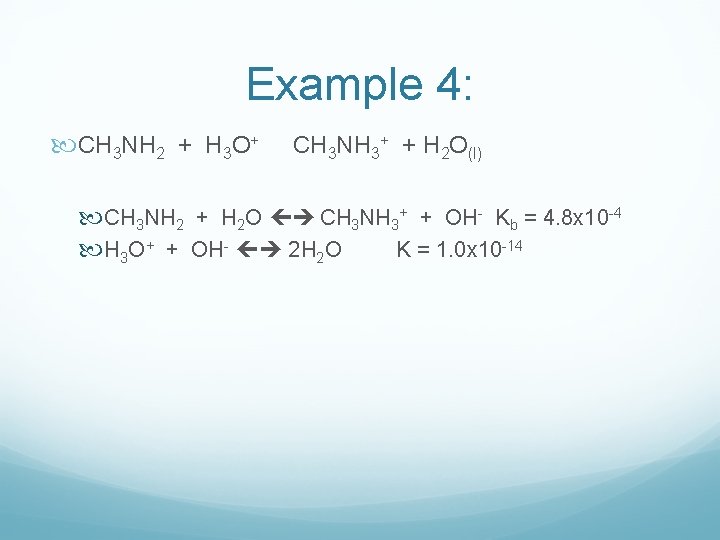
Example 4: CH 3 NH 2 + H 3 O+ CH 3 NH 3+ + H 2 O(l) CH 3 NH 2 + H 2 O CH 3 NH 3+ + OH- Kb = 4. 8 x 10 -4 H 3 O+ + OH- 2 H 2 O K = 1. 0 x 10 -14

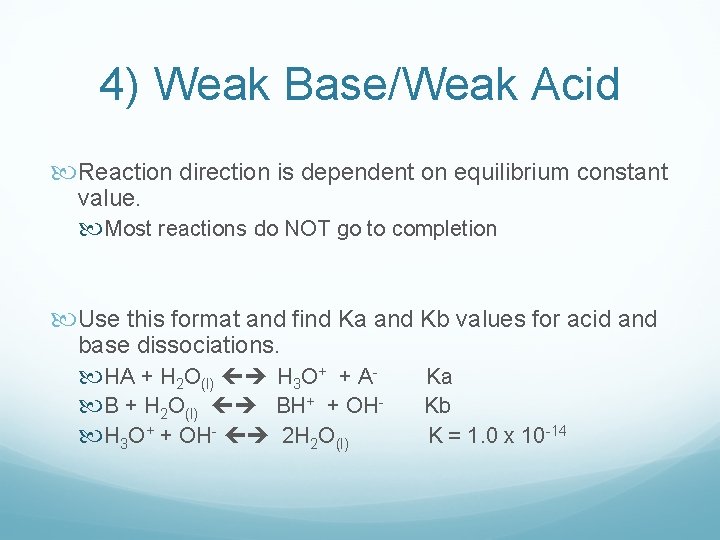
4) Weak Base/Weak Acid Reaction direction is dependent on equilibrium constant value. Most reactions do NOT go to completion Use this format and find Ka and Kb values for acid and base dissociations. HA + H 2 O(l) H 3 O+ + A B + H 2 O(l) BH+ + OH H 3 O+ + OH- 2 H 2 O(l) Ka Kb K = 1. 0 x 10 -14

Example 5: CH 3 NH 2 + HNO 2 CH 3 NH 2+ + NO 2 Krxn = (Ka) (Kb)(1 x 1014) = 2. 5 x 107

Homework pp. 670 #63 -64, 67 -70