Sali Mark OSCC Investigator Meeting Salivary Transcriptome Markers










- Slides: 14

Sali. Mark™ OSCC Investigator Meeting Salivary Transcriptome Markers for Oral Squamous Cell Carcinoma Detection Sali. Mark™ OSCC

Study Organization Sponsor: Peri. Rx, LLC Chief Medical Office – Dr. Jack Martin CEO – Stephen M. Swanick PI: Dr. Marc Surkin Statistician/Regulatory Consultant: Dr. Richard Chiacchierini Former Director FDA IVD Sali. Mark™ OSCC

NIH & Salivary Dx: Vision & Investments By 2013, determine the efficacy of using salivary diagnostics to monitor health and diagnose at least one systemic disease. Sali. Mark™ OSCC

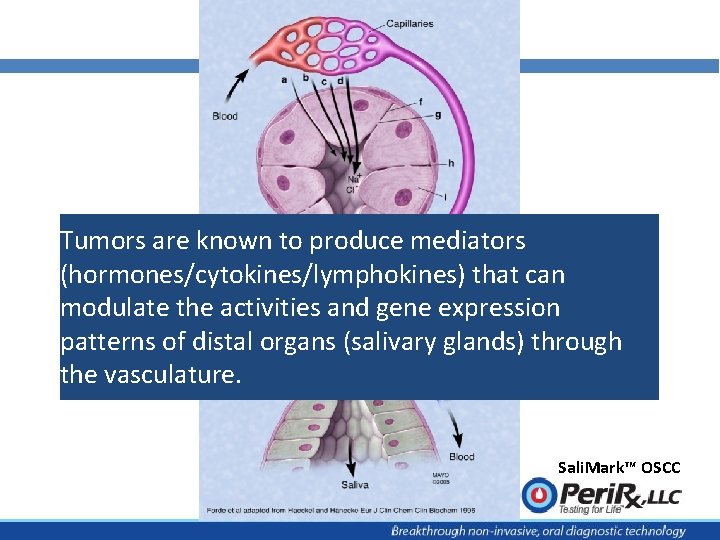
Tumors are known to produce mediators (hormones/cytokines/lymphokines) that can modulate the activities and gene expression patterns of distal organs (salivary glands) through the vasculature. Sali. Mark™ OSCC

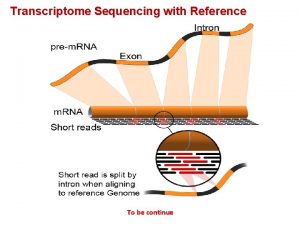
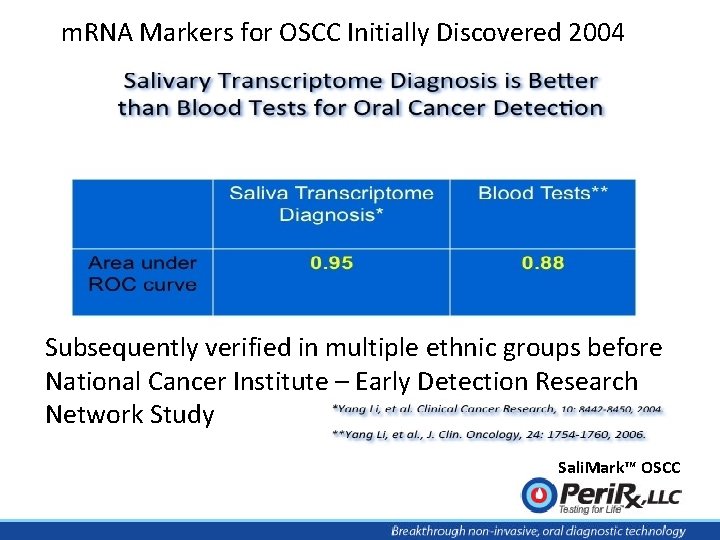
m. RNA Markers for OSCC Initially Discovered 2004 Subsequently verified in multiple ethnic groups before National Cancer Institute – Early Detection Research Network Study Sali. Mark™ OSCC

395 subjects studied by National Cancer Institute – Early Detection Research Network Conclusions: The validation of these biomarkers showed their feasibility in the discrimination of OSCCs A prospective blinded trial was necessary Sali. Mark™ OSCC

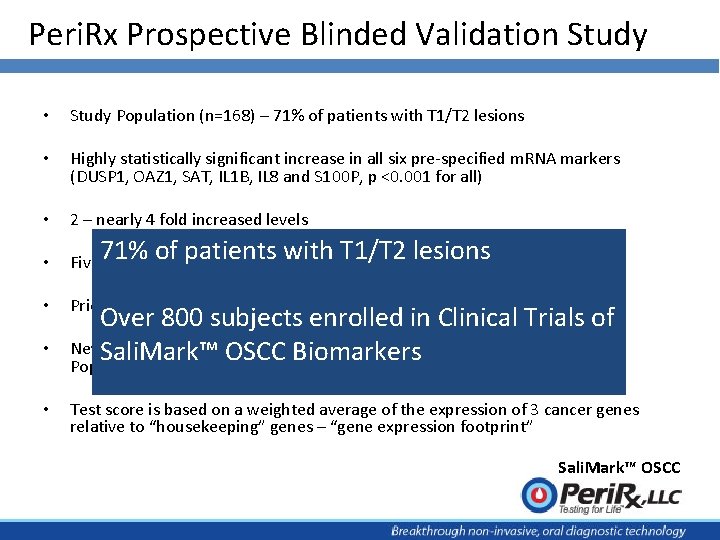
Peri. Rx Prospective Blinded Validation Study • Study Population (n=168) – 71% of patients with T 1/T 2 lesions • Highly statistically significant increase in all six pre-specified m. RNA markers (DUSP 1, OAZ 1, SAT, IL 1 B, IL 8 and S 100 P, p <0. 001 for all) • 2 – nearly 4 fold increased levels • Five of 6 m. RNAs significantly upregulated in dysplasia • Prior NCI Multi-marker Model Validated • New Highly Discriminatory Multi-marker Models Developed in Intended Use Population of Patients with Suspicious Oral Lesions • Test score is based on a weighted average of the expression of 3 cancer genes relative to “housekeeping” genes – “gene expression footprint” Sali. Mark™ OSCC

Peri. Rx Prospective Blinded Validation Study • Study Population (n=168) – 71% of patients with T 1/T 2 lesions • Highly statistically significant increase in all six pre-specified m. RNA markers (DUSP 1, OAZ 1, SAT, IL 1 B, IL 8 and S 100 P, p <0. 001 for all) • 2 – nearly 4 fold increased levels • of patients T 1/T 2 lesions Five 71% of 6 m. RNAs significantly with upregulated in dysplasia • Prior NCI Multi-marker Model Validated • • Over 800 subjects enrolled in Clinical Trials of New. Sali. Mark™ Highly Discriminatory Multi-marker Models Developed in Intended Use OSCC Biomarkers Population of Patients with Suspicious Oral Lesions Test score is based on a weighted average of the expression of 3 cancer genes relative to “housekeeping” genes – “gene expression footprint” Sali. Mark™ OSCC

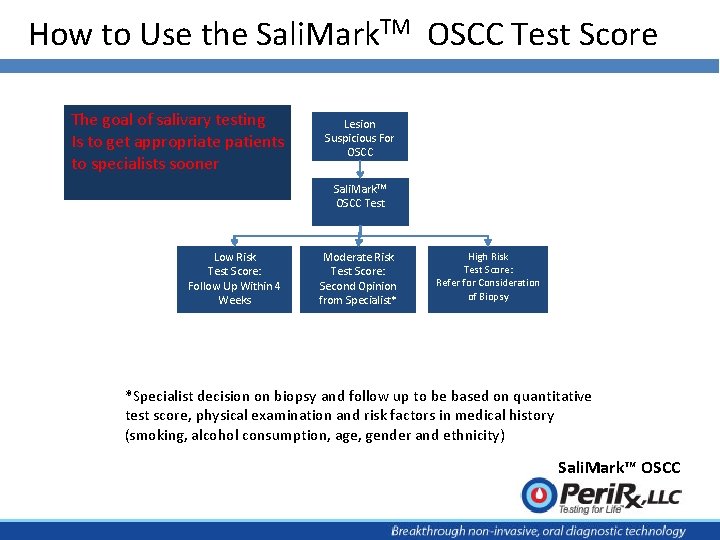
How to Use the Sali. Mark. TM OSCC Test Score The goal of salivary testing Is to get appropriate patients to specialists sooner Lesion Suspicious For OSCC Sali. Mark. TM OSCC Test Low Risk Test Score: Follow Up Within 4 Weeks Moderate Risk Test Score: Second Opinion from Specialist* High Risk Test Score: Refer for Consideration of Biopsy *Specialist decision on biopsy and follow up to be based on quantitative test score, physical examination and risk factors in medical history (smoking, alcohol consumption, age, gender and ethnicity) Sali. Mark™ OSCC

Dual Approval Pathway - We Need Additional Cases! Current data supports CLIA Approved Laboratory Developed Test Additional cases necessary to support FDA submission Sali. Mark™ OSCC

Getting started Online CITI Program training course on ethical issues in research required by IRB for one investigator in each practice Sign financial disclosure statement Sign contract with Peri. Rx, LLC Sali. Mark™ OSCC

Exclusion Criteria Inclusion Criteria 18 years and older Oral lesion requiring biopsy Recently diagnosed OSCC not as yet treated Diagnosis of cancer within the last 5 years, excluding non-melanoma skin cancer or OSCC within 2 years If > 5 years since other cancer diagnosis, must be free of known disease & not on current treatment for cancer Enrollment as easy as spitting in a cup Prior Immunosuppressive therapy (in last 2 months) or current autoimmune disorder Enrollment Tips HIV or Hepatitis (in last 2 months) Review practice schedule on Fridays for potential cases Alert Priority Express Courier at 610 -364 -3300 (Available 24/7/365) OR Email if scheduled in advance at customerservice@priorityexpress. com Account Name: Peri. Rx, Account # 10277 Physician support for the importance of this trial is key to successful enrollment Stress the noninvasive/painless nature of the study, the absence of risk and the benefits Sali. Mark™ OSCC Just Do It! – once you enroll the first patient the rest is easy

Saliva Collection Steps • The patient should fast for 1 hour • Have the patient rinse their mouth with water • Collect 2 cc of saliva into Falcon tube (to top of cone section) • Label the tube with provided waterproof label • The specimen is identified by the study ID (for example - Surkin 001, 002, etc. ) • Place the capped tube in bag with ice • Call the courier service Sali. Mark™ OSCC

Conclusions We Need Additional Subjects for an FDA Submission Sali. Mark™ OSCC
 [email protected]
[email protected] Investigator meeting services
Investigator meeting services Oncocytoma salivary gland
Oncocytoma salivary gland Oncocytoma salivary gland
Oncocytoma salivary gland Importance of bile juice in digestion
Importance of bile juice in digestion Weber glands
Weber glands Colon
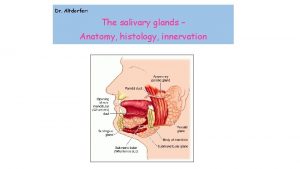
Colon Salivary gland disease classification
Salivary gland disease classification Submandibular gland excision
Submandibular gland excision Warthin's tumor
Warthin's tumor Xerotrachea
Xerotrachea Submadibular
Submadibular Intestinal structure
Intestinal structure Function of salivary glands
Function of salivary glands Minor salivary glands
Minor salivary glands