SALADIN CH 24 Fluid Electrolyte AcidBase Balance Fluid

SALADIN CH. 24 Fluid, Electrolyte, & Acid-Base Balance
![Fluid Compartments Fluid occupies two main compartments. [55 -60% body weight] Intracellular fluid (ICF) Fluid Compartments Fluid occupies two main compartments. [55 -60% body weight] Intracellular fluid (ICF)](http://slidetodoc.com/presentation_image_h/2e1d77798b0920fc25275da56de4af39/image-2.jpg)
Fluid Compartments Fluid occupies two main compartments. [55 -60% body weight] Intracellular fluid (ICF) – about two thirds by volume, contained in cells = 65% of body fluids Extracellular fluid (ECF) – consists of two major subdivisions = 35% Plasma – the fluid portion of the blood. Interstitial fluid (IF) – fluid in spaces between cells.
Fluid Compartments Other fluids. [lymph, CSF, GI fluids, synovial fluid, ocular humors, pleural, pericardial and peritoneal fluids, glomerular filtrate]
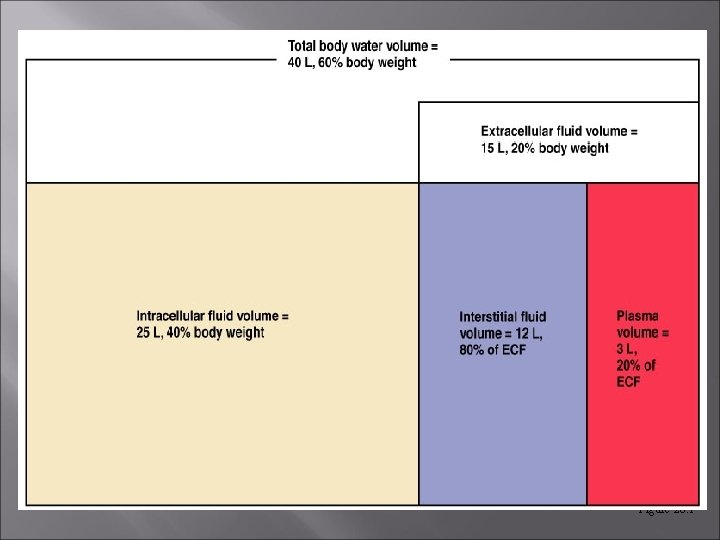
Figure 26. 1

Fluid Movement Among Compartments Water moves by osmosis which is determined by electrolyte movement & balance

Water Balance & ECF Osmolality For proper hydration, water intake must equal water output. Total ingested water [2500 m. L/day] Metabolic [from aerobic resp. and dehydration synthesis] [200 m. L/day] Food & drink – [2300 m. L]
Water Balance & ECF Osmolality Average outputs – increase with activity, environment, etc. Total output [2500 m. L] Kidneys – urine [1500 m. L/day] Skin & sweat – 500 m. L/day Lungs GI – esp. [300 m. L/day] tract – feces [200 m. L/day]
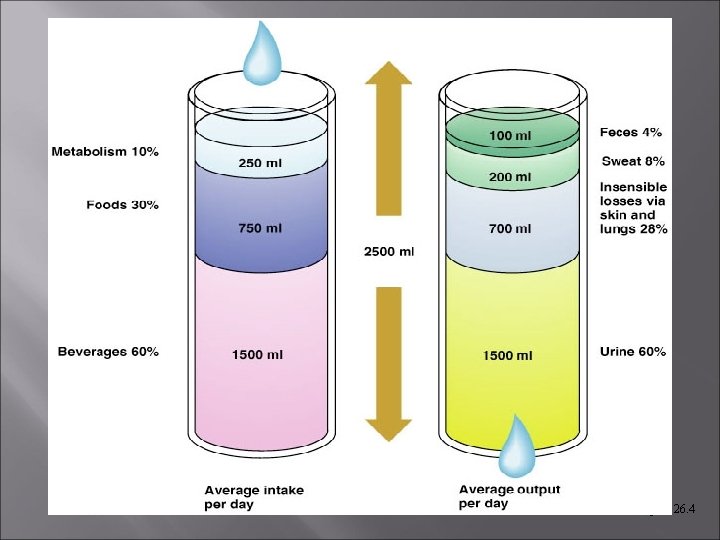
Figure 26. 4
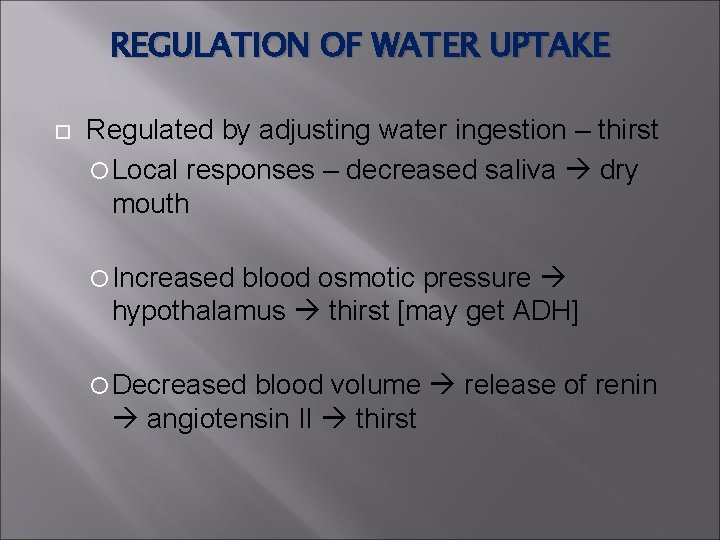
REGULATION OF WATER UPTAKE Regulated by adjusting water ingestion – thirst Local responses – decreased saliva dry mouth Increased blood osmotic pressure hypothalamus thirst [may get ADH] Decreased blood volume release of renin angiotensin II thirst
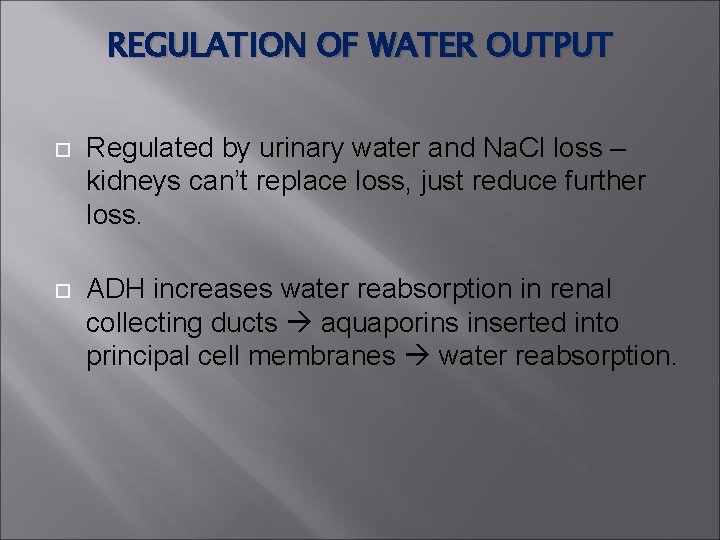
REGULATION OF WATER OUTPUT Regulated by urinary water and Na. Cl loss – kidneys can’t replace loss, just reduce further loss. ADH increases water reabsorption in renal collecting ducts aquaporins inserted into principal cell membranes water reabsorption.

Disorders of Water Balance: Dehydration Water loss exceeds water intake & the body is in negative fluid balance. Causes include: hemorrhage, severe burns, prolonged vomiting or diarrhea, profuse sweating, water deprivation, & diuretic abuse.

Disorders of Water Balance: Dehydration – get increased osmolarity; Diabetes mellitus & insipidus, overuse of diuretics, diarrhea; [can lead to shock] Use isotonic salts solutions for replacement of extreme fluid loss – prevents hypotonic swelling.
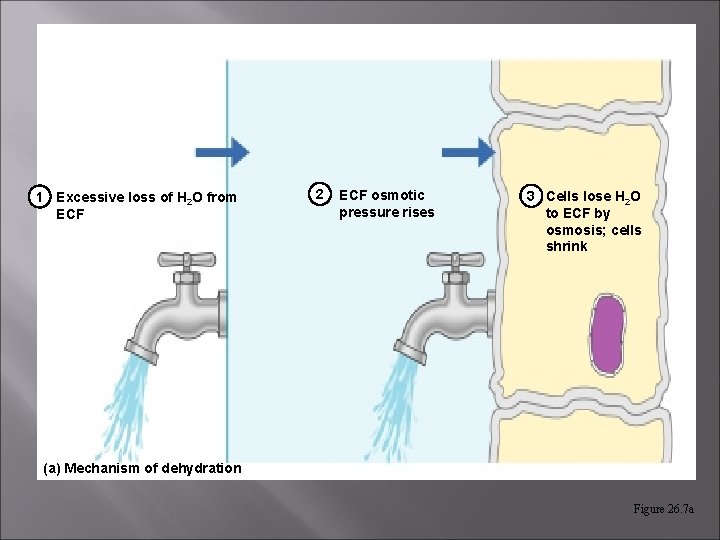
1 Excessive loss of H 2 O from ECF 2 ECF osmotic pressure rises 3 Cells lose H 2 O to ECF by osmosis; cells shrink (a) Mechanism of dehydration Figure 26. 7 a

Disorders of Water Balance: Fluid Excess Water intoxication – take in faster than can remove – hypotonic ECF Hyponatremia] cell swelling and cerebral edema
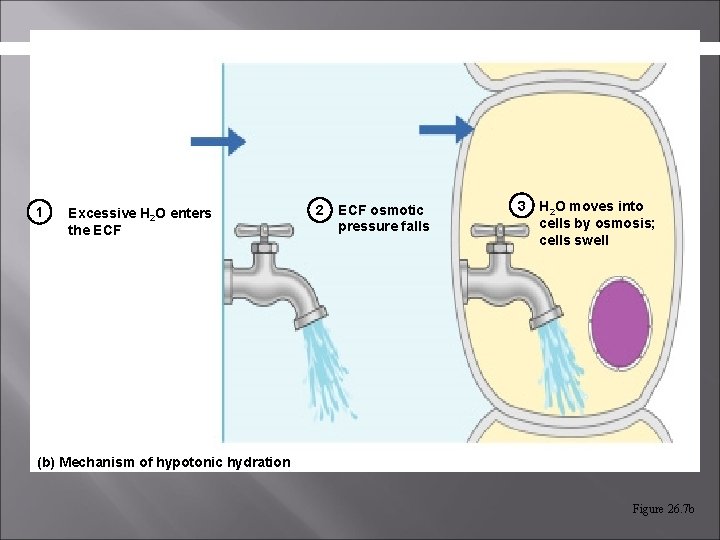
1 Excessive H 2 O enters the ECF 2 ECF osmotic pressure falls 3 H 2 O moves into cells by osmosis; cells swell (b) Mechanism of hypotonic hydration Figure 26. 7 b
Disorders of Water Balance: Edema Atypical accumulation of fluid in interstitial spaces, leading to tissue swelling. Caused by anything that increases flow of fluids out of the bloodstream or hinders their return. Increases blood pressure. May involve vessel blockage, CHF.

Electrolyte Balance Electrolyte - substance that dissociates into cations & anions in water & conducts electricity in solution.
Functions of Electrolytes Essential minerals - enzyme cofactors. Control of osmosis. Maintenance of acid/base balance. Conduction of electrical current.
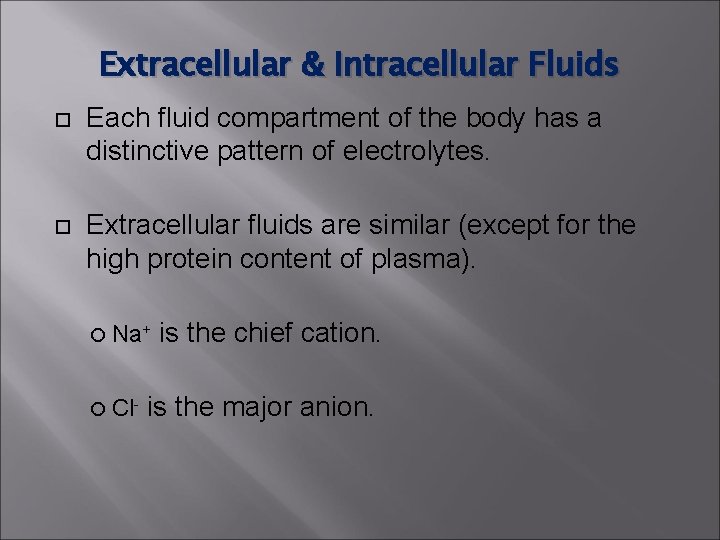
Extracellular & Intracellular Fluids Each fluid compartment of the body has a distinctive pattern of electrolytes. Extracellular fluids are similar (except for the high protein content of plasma). Na+ Cl- is the chief cation. is the major anion.
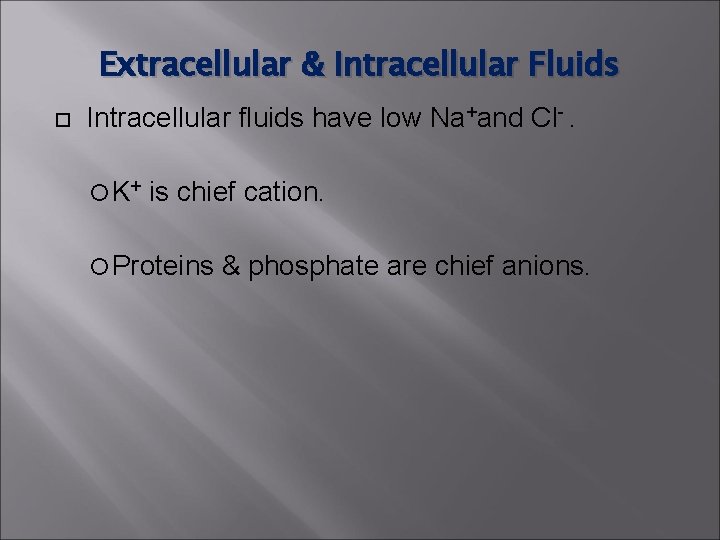
Extracellular & Intracellular Fluids Intracellular fluids have low Na+and Cl-. K+ is chief cation. Proteins & phosphate are chief anions.
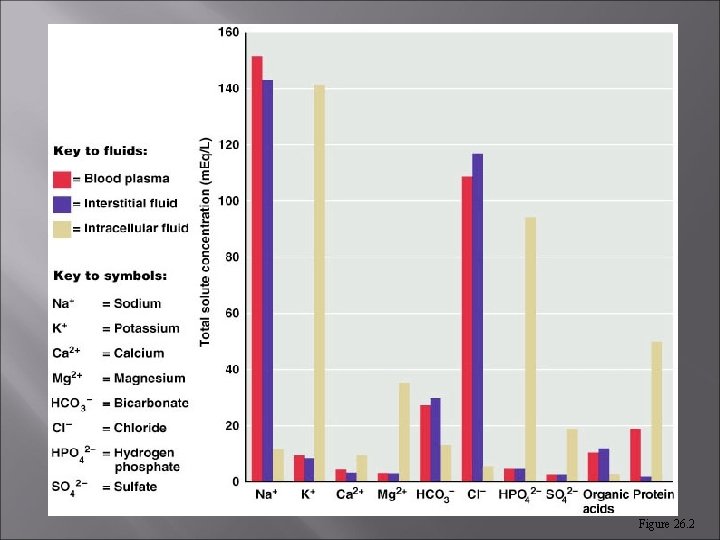
Figure 26. 2
Sodium & Electrolyte Balance Na+ holds central position in fluid & electrolyte balance. [Plasma levels = 142 m. Eq/L] Sodium salts account for 90 of all solutes in the ECF. Na+ is the only cation exerting significant osmotic pressure - most important solute in determining water distribution.
Sodium & Electrolyte Balance Adult need 0. 5 g/day – in America always have EXCESS – problem is getting rid of it Concentrations are maintained by maintaining water levels – “water follows salt” 65% of Na+ in renal filtrate is automatically reabsorbed.
Regulation of Sodium Balance: Levels regulated by: Aldosterone - [increases reabsorption of Na+ and excretion of K+], ascending loop, DCT & collecting ducts ADH [increases water reabsorption in response to increases in Na] ANP [increases Na+ excretion by kidneys by inhibing ADH & aldosterone]
Regulation of Sodium Balance: Estrogens – enhances Na reabsortion and water retention Progesterone may decrease Na+ reabsorption by blocking aldosterone. Glucocorticoids – can cause edema

Na+ Imbalances Hypernatremia water retention, hypertension & edema Hyponatremai hypotonic hydration if not corrected
K+ and Electrolyte Balance Most abundant intracellular cation. Functions in impulse conduction, OP regulation, protein synthesis & p. H. Levels regulated in the kidneys by aldosterone.

Influence of Aldosterone stimulates potassium ion secretion by “principal cells. ” In collecting ducts, for each Na+ reabsorbed, a K+ is secreted. Increased K+ in ECF around adrenal cortex causes release of aldosterone & potassium secretion.
K+ Imbalances Hyperkalmia – get different responses if fast or slow onset. Fast - The heart is really sensitive to too much [can cause membrane depolarization - hyperexcitability]; Slow – cells become LESS excitable Hypokalmia - too little [causes hyperpolarization and non-responsiveness].
Regulation of Chloride Cl- is the major anion accompanying Na+ in the ECF. Functions in OP regulation, HCl formation. Levels regulated by Na+ movements.
Regulation of Calcium & Phosphate Calcium-most abundant mineral. Abundant extracellular cation. Functions in bone, teeth, blood clotting, impulse conduction, muscle contraction.
Regulation of Calcium & Phosphate Levels controlled by: PTH Calcitrol Calcitonin
Regulation of Calcium & Phosphate Hypercalcemia – from alkalosis or hypothyroidism – inhibits depolarization weakness, arrhythmia Hypocalcemia – Vit. D deficiency, lactation, pregnancy, etc. Too little can increase excitability & may lead to tetanus;
Regulation of Calcium & Phosphate PHOSPHATE Functions: Nucleic acids, ATP, phospholipids, bone, etc. Control: Kidneys reabsorb if levels drop, Parathyroid hormone stimulates excretion
Acid-Base Balance Normal p. H of blood is 7. 35 – 7. 45. 3 mechanisms to maintain proper p. H: Buffer systems Changes in respiration Excretion by kidney
![Acid-Base Balance Acid = proton [H+] donor; . Most H+ originates as metabolic products. Acid-Base Balance Acid = proton [H+] donor; . Most H+ originates as metabolic products.](http://slidetodoc.com/presentation_image_h/2e1d77798b0920fc25275da56de4af39/image-38.jpg)
Acid-Base Balance Acid = proton [H+] donor; . Most H+ originates as metabolic products. Strong Weak dissociates completely acid only dissociates a little Base = proton acceptor Strong – binds a lot, Weak binds less
Chemical Buffer Systems Buffers resist changes in p. H when strong acid or strong base are added. Weak acid + salt of that acid. Works by taking up or releasing H+ ions to maintain p. H at a given level. Three major chemical buffer systems: Bicarbonate/carbonic acid buffer system
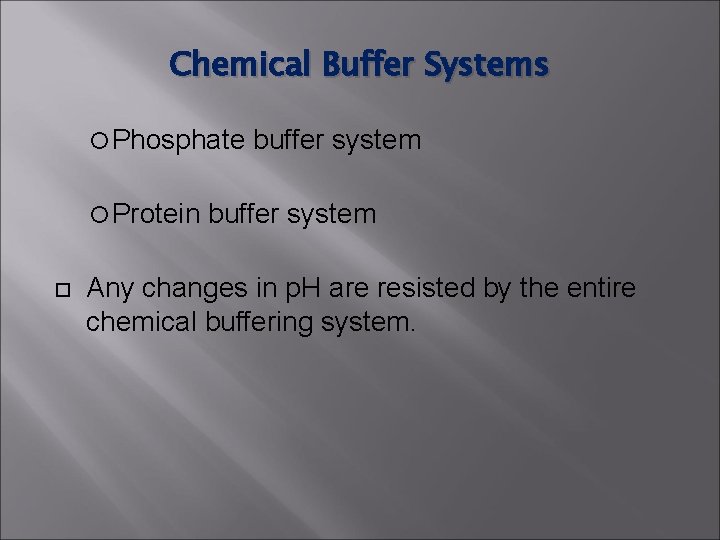
Chemical Buffer Systems Phosphate Protein buffer system Any changes in p. H are resisted by the entire chemical buffering system.
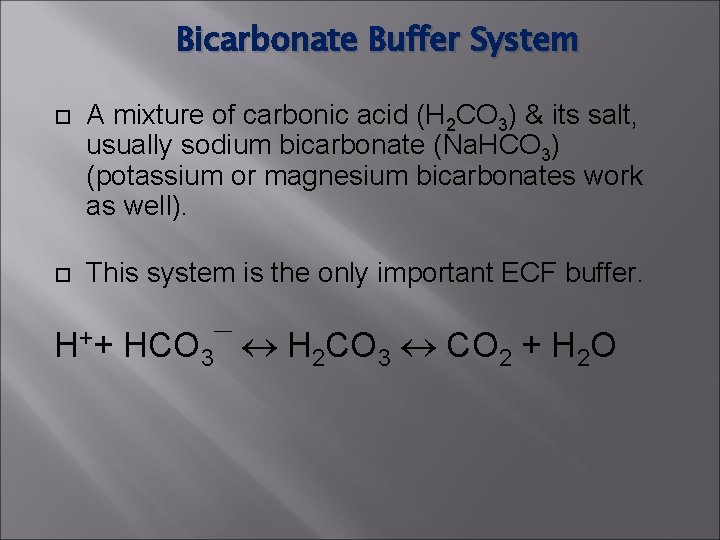
Bicarbonate Buffer System A mixture of carbonic acid (H 2 CO 3) & its salt, usually sodium bicarbonate (Na. HCO 3) (potassium or magnesium bicarbonates work as well). This system is the only important ECF buffer. H++ HCO 3¯ H 2 CO 3 CO 2 + H 2 O
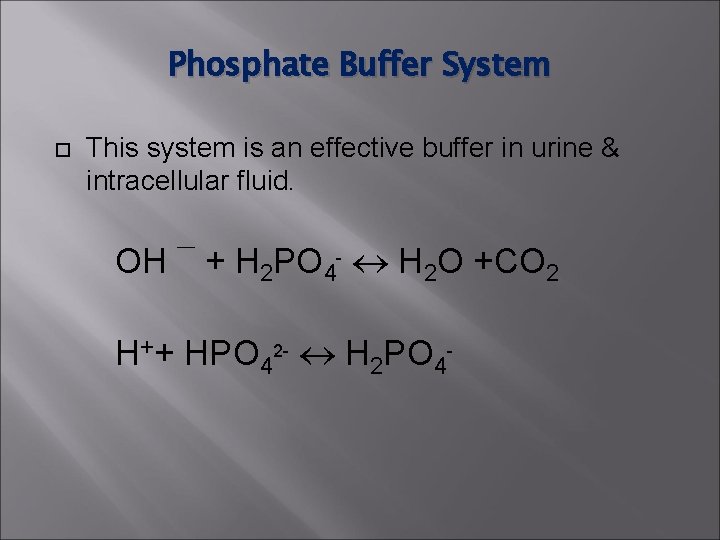
Phosphate Buffer System This system is an effective buffer in urine & intracellular fluid. OH ¯ + H 2 PO 4 - H 2 O +CO 2 H++ HPO 42 - H 2 PO 4 -
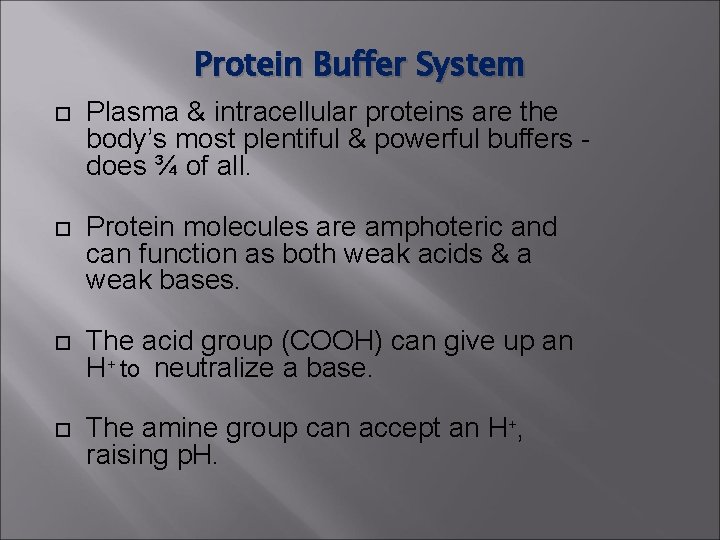
Protein Buffer System Plasma & intracellular proteins are the body’s most plentiful & powerful buffers does ¾ of all. Protein molecules are amphoteric and can function as both weak acids & a weak bases. The acid group (COOH) can give up an H+ to neutralize a base. The amine group can accept an H+, raising p. H.

Respiration The respiratory system regulation of acid-base balance is a physiological buffering system. Exhalation of CO 2 lowers H+ by lowering carbonic acid With alkalosis – get shallow slow breathing to help ACCUMULATE CO 2

Kidneys can expel H’s by secretion – neutralize it in tubules with bicarb, phosphate, etc. Only way to remove non-carbonic acid H+ [phosphoric uric, lactic acid, ketone bodies] Also only way to regulate alkaline substances – can break down bicarb to CO 2 and reabsorb that.
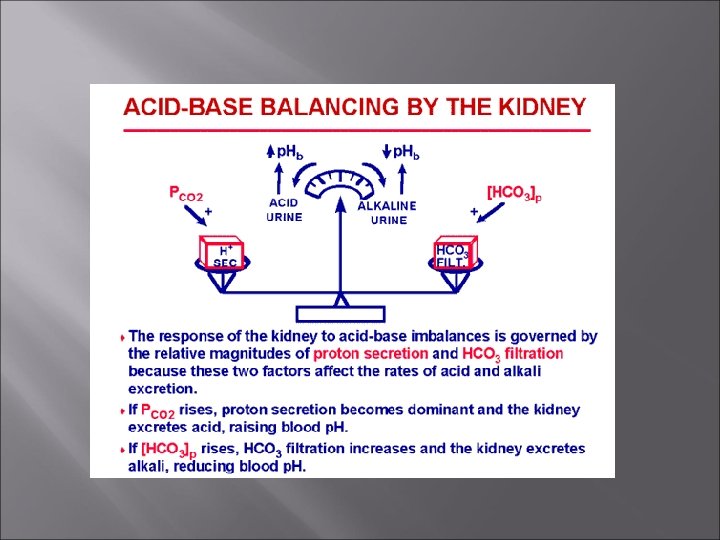

Blood p. H Acidosis: p. H below 7. 35 Alkalosis: p. H above 7. 45 Compensation-physiological changes that occur to bring p. H back to normal.
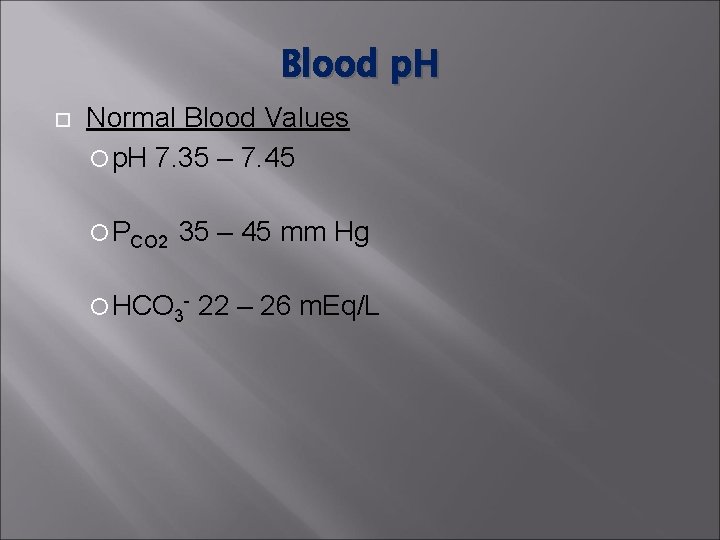
Blood p. H Normal Blood Values p. H 7. 35 – 7. 45 PCO 2 35 – 45 mm Hg HCO 3 - 22 – 26 m. Eq/L
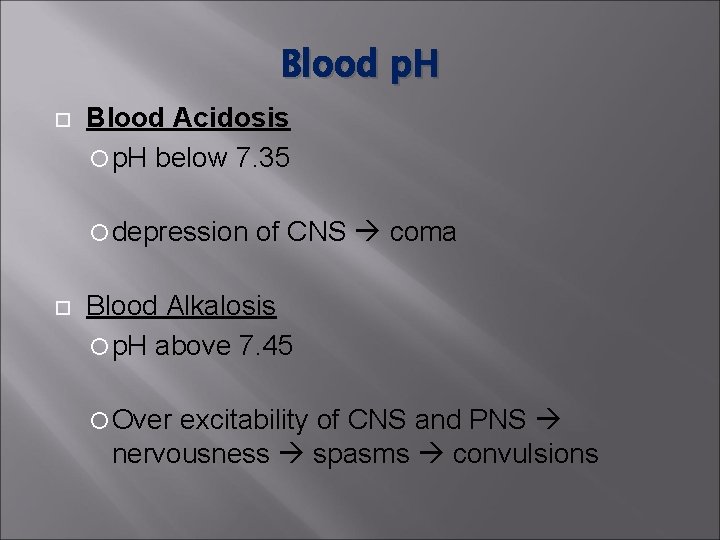
Blood p. H Blood Acidosis p. H below 7. 35 depression of CNS coma Blood Alkalosis p. H above 7. 45 Over excitability of CNS and PNS nervousness spasms convulsions

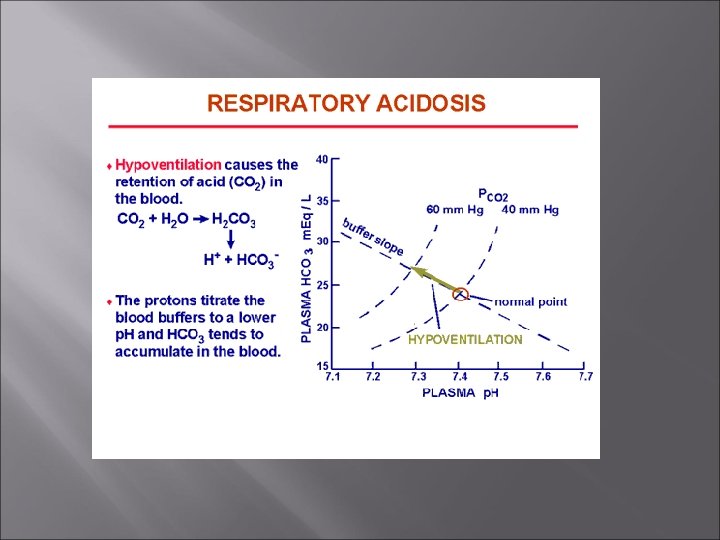
Respiratory Acidosis and Alkalosis Respiratory acidosis is the most common cause of acid-base imbalance. Occurs when a person breathes shallowly, or gas exchange is hampered by diseases such as pneumonia, cystic fibrosis, or emphysema.

Respiratory Acidosis and Alkalosis Acidosis Elevated p. CO 2, low p. H Compensation – renal H+ secretion, increased bicarbonate reabsorption Treatment – respiratory and IV bicarbonate
Respiratory Acidosis and Alkalosis Low p. CO 2, high p. H Caused by hyperventilation [e. g. From oxygen deficiency] Compensation – renal – decrease H+ secretion, bicarbonate elimination Treatment – breathe into a bag
Metabolic Acidosis Low systemic and arterial bicarbonate. Caused by loss of bicarbonate, accumulation of another acid or kidney failure. Compensation – respiratory hyperventilation [increased rate and depth]. PCO 2 falls. Treatment – Na. HCO 3 IV
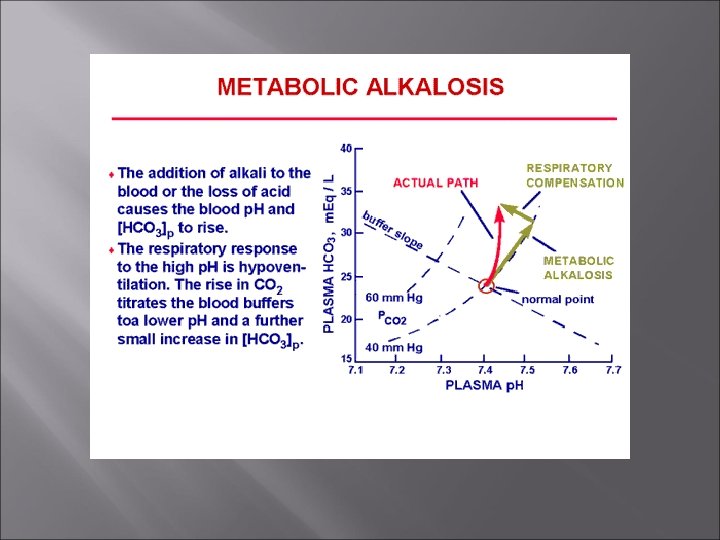
Metabolic Alkalosis Rising blood p. H and bicarbonate levels indicate metabolic alkalosis. Typical causes are loss of acid (e. g vomiting), intake of excess base (e. g. , from antacids), Compensation – respiratory – hypoventilation [slow, shallow]. PCO 2 rises. Treatment – electrolyte/fluid therapy
- Slides: 56