Safety Talk Mixing Incompatible Chemicals Nitric acid organic

Safety Talk Mixing Incompatible Chemicals (Nitric acid + organic solvent) Inhong Hwang February 25 th, 2019

Incompatible materials should not be stored together where they can be inadvertently mixed or where a spill or leak can cause danger. 1. Oxygen vs fuels 2. Water reactive materials vs flammables 3. Strong acids vs bases 4. Materials which can produce poisonous gases vs products which accelerate the release of the gas (Example: chlorine vs ammonia) 5. Explosives (picric acid, etc. ) vs fuels 6. Incompatible acids (Examples: perchloric acid vs sulfuric acid, nitric acid vs acetic acid)

Nitric acid + organic solvent “…Nitric acid is the common chemical most frequently involved in reactive incidents, and this is a reflection of its exceptional ability to function as an effective oxidant even under fairly dilute conditions (unlike sulfuric acid) or an ambient temperature (unlike perchloric acid). Its other notable ability to oxidize most organic compounds to gaseous carbon dioxide, coupled with its own reduction to gaseous ‘nitrous fume’ has been involved in many incidents in which closed, or nearly closed reaction vessels or storage cabinets have failed from internal gas pressure. ” (Bretherick 1990) Nitric acid must be physically segregated from organic solvents as well as all other acids since it is in a class by itself.
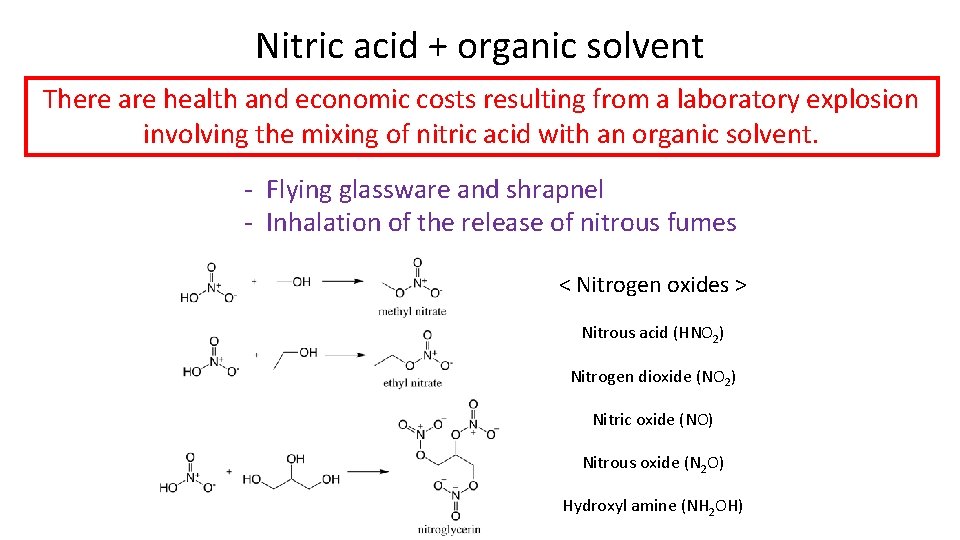
Nitric acid + organic solvent There are health and economic costs resulting from a laboratory explosion involving the mixing of nitric acid with an organic solvent. - Flying glassware and shrapnel - Inhalation of the release of nitrous fumes < Nitrogen oxides > Nitrous acid (HNO 2) Nitrogen dioxide (NO 2) Nitric oxide (NO) Nitrous oxide (N 2 O) Hydroxyl amine (NH 2 OH)

In the 1980’s, UCSB A strong oxidizing acid (nitric acid) and organic solvents were mixed inside a waste container. December 19, 2014, Georgetown University

Avoid mixing nitric acid waste with : - Acetic acid Acetic anhydride Acetonitrile Acrylonitrile Alcohols - Aldehydes Alkali metals Ammonia Cyanides Powdered metals Organic matter - Prohibit the reuse of an empty organic solvent bottle to collect nitric waste. - A standard operating procedure recommend that an organic solvent bottle should be triple rinsed with water.
- Slides: 6