Safety in an Organic Synthesis Lab 1 THE

Safety in an Organic Synthesis Lab 1

THE CHEMISTRY LABORATORY INCLUDES HAZARDS AND RISKS. This presentation assumes your knowledge of the safety rules for an introductory organic laboratory. If you did not take organic chemistry (231 L/232 L) here, you should review the information in the attached link: Organic Chemistry Lab Safety 2

1. PPE Personal Protective Equipment: What must be worn when you work in the laboratory. Eye Protection Lab Coat Long Pants Closed Toed Shoes and Socks– no exposed skin around feet Lab gloves – when required 3

Eye Protection • Contact lenses are OK as long as glasses/goggles are worn • Prescription glasses – you must wear goggles over them • Safety goggles are provided in organic labs in UV irradiating cabinets • Eye wash stations are present in all labs 4

Clothing and Foot Protection • Clothing must cover all exposed skin including legs/ankles • Socks as required PPE • Stockings or leggings do not provide good coverage • Sandals, flip-flops, Crocs, opentoe and open-top (i. e. ballet flat) shoes and canvas shoes (i. e. Toms) are not appropriate. These are not going to protect your feet if you drop a piece of glass with a liquid chemical reagent in it. 5

Hand Protection: Chemically resistant Lab Gloves ✓ • Wear gloves of a material known to be resistant to permeation by the substances in use – nitrile is good for most of our laboratory classes. • Inspect each glove for small holes or tears before use. • When you spill on your glove or tear it, change it immediately. Throw gloves away any time you take them off. 6

Use of Gloves Remove gloves before handling objects such as doorknobs, telephones, pens, computer keyboards, p. H meter or other electronic buttons, or phones while in lab. It might be convenient to have one gloved hand one ungloved hand to do procedures where these kinds of things are used. • Throw away gloves anytime you take them off. • You should expect to use several pairs of gloves in any given lab period. • Glove video (Review this video if necessary) 7

Glove Recycling Contaminated Gloves with visible signs of chemical exposure or those used with hazardous substances should be collected in solid waste or biohazard containers. Uncontaminated Gloves that have no visible sign of chemical exposure or residue can be placed in the glove recycling container. If there’s any questions or the boxes need attention, please contact Dr. Katherine Mullaugh. Email: mullaughkm@cofc. edu Phone: 843. 953. 6587 8

2. Safety Equipment in the Lab Eyewash and Safety Shower: Know where these are in your lab. 9

Eyewash / Safety Shower The eyewash is on the left. Pull the handle and a fountain of water will appear that you can use to bathe your eyes. The safety shower is on the right. Pull the handle and water will start spraying from the shower head on the ceiling. There’s no drain in the floor – we only do this in emergencies, because a flood of water will have to be cleaned up. 10

3. Chemical Fume Hoods: You must do your experiment in the hood if any of your reagents are flammable, have harmful fumes or present a splash or explosion hazard. This means pretty much at all times for organic chemists. 11

Using the Fume Hoods properly This window/bar is called the sash. If this is not saying NORMAL, then the hood is not protecting you. Keeping the sash and sliding panels in proper position keeps this NORMAL, otherwise the alarm goes off. If the alarm goes off, you need to reposition things to the correct positions, then press the “mute” button to reset the controller. The sash should never be raised above the green “operation” level when you 12 are working in the hood.
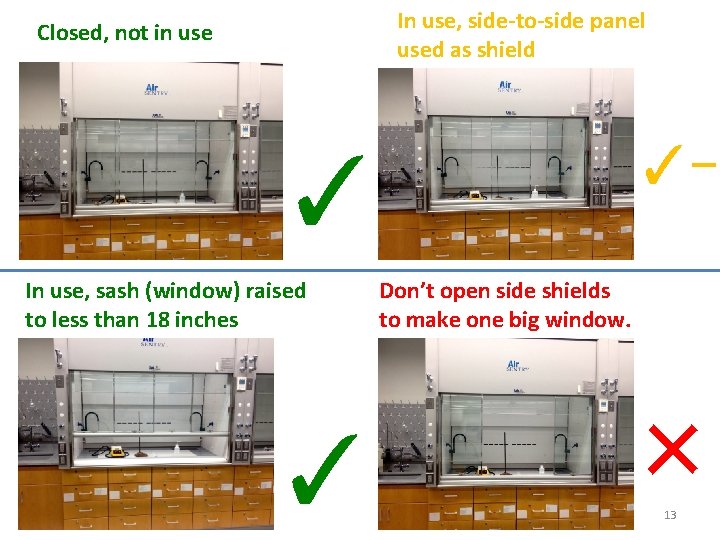
In use, side-to-side panel used as shield Closed, not in use ✓- ✓ In use, sash (window) raised to less than 18 inches ✓ Don’t open side shields to make one big window. × 13

• BEFORE USING THE HOOD: MAKE SURE IT IS NEAT & CLEAN & UNCLUTTERED. • When using a laboratory hood, Check that the airflow is in the normal range on the digital display • Turn on the hood light • Set the equipment and chemicals back at least 6 inches. • Never lean in and/or put your head in the hood when you are working. This is worse than doing the experiment with no hood at all. http: //web. princeton. edu/sites/ehs/labsafetymanual/SOC. htm ehs. unc. edu/training/self_study/fume_hood/docs/fume_hood. ppt 14

Know the risks of the chemical reagents you are working with and the procedures you are going follow. Unlike the introductory organic laboratory the chemicals used in this course will be dangerous, at times with unknown health risks. Unlike the introductory organic laboratory, the procedures will be dangerous, at times very dangerous. 15

Labels are important Even if it seems obvious. In the chemistry lab, nothing is ever obvious. 16
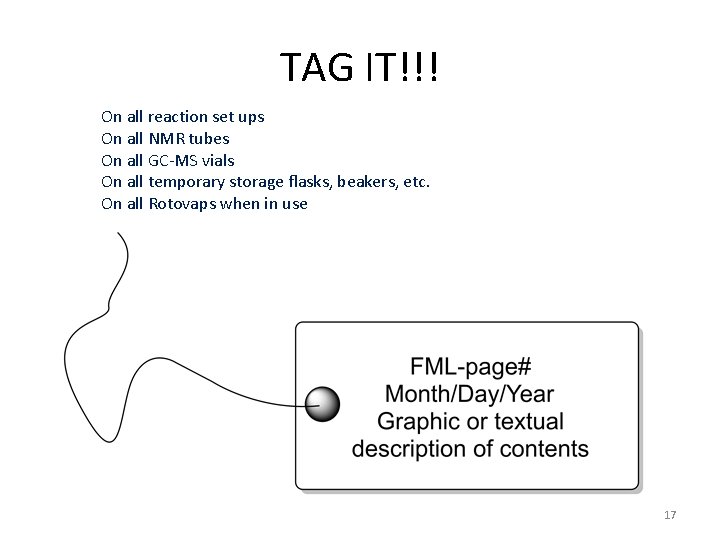
TAG IT!!! On all reaction set ups On all NMR tubes On all GC-MS vials On all temporary storage flasks, beakers, etc. On all Rotovaps when in use 17
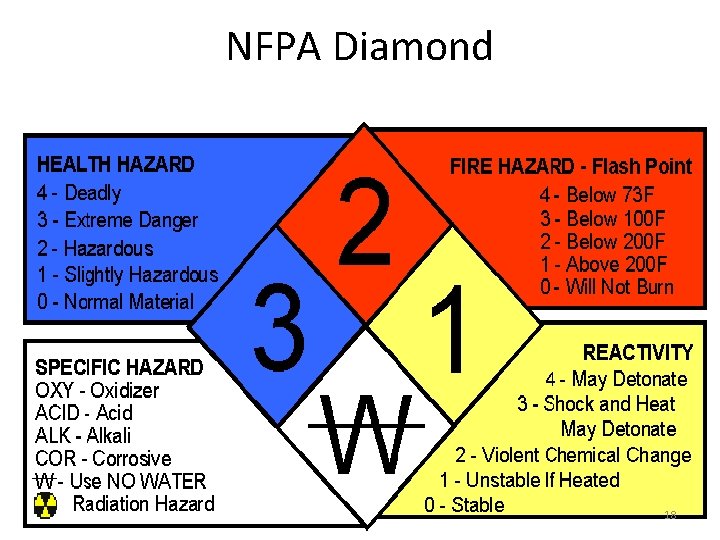
NFPA Diamond 18

NFPA Label For Intermediate or Long Term Use • On products, once characterized. • On solvent solutions prepared for chromatographic separations. • On acid or base solutions. • On reagents transferred into secondary containers. • On anything that will be in the lab that does not have a primary label from the manufacturer or a string tag label you put on it. 19

Where to Find NFPA and Safety Information? From MSDS sheets – on OAKS – on manufacturer website – hard copies in the lab – ask instructor if you cannot locate the MSDS you need 20
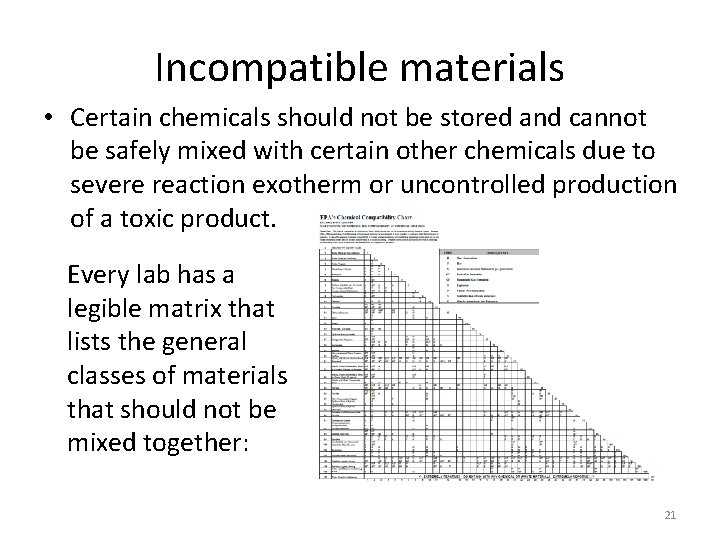
Incompatible materials • Certain chemicals should not be stored and cannot be safely mixed with certain other chemicals due to severe reaction exotherm or uncontrolled production of a toxic product. Every lab has a legible matrix that lists the general classes of materials that should not be mixed together: 21

Regulatory agencies and standards • • Over the last 40 years the US and state governments and various international bodies have developed regulations and standards that try to improve safety and industrial hygiene standards including the following: EPA: Environmental Protection Agency, who have the primary responsibility to ensure chemicals are used and disposed of in an environmentally sensitive manner TOSCA: the Toxic Substances Control Act of 1976 regulates which chemicals may be produced or imported in the US OSHA: Occupational Safety and Health Administration is the US agency that assures safe and healthful working conditions by setting and enforcing standards NIOSH: National Institute for Occupational Safety and Health is responsible for researching the prevention of work-related injury and illness, and providing guidance to OSHA RCRA: the resource conservation and recovery act of 1976 that sets the standards for chemical waste disposal in this country and oversees the “superfund law” CERCLA California Proposition 65: The state of California passed a very rigorous law to protect drinking and ground water from toxic chemicals. It is increasingly the standard for companies when evaluating chemical safety All of these regulations have been developed to make the use and handling of chemical safer, and their impact on lab safety has been profound. 22

Acute and chronic toxicology • Acute toxin: rapid absorption of the substance and the exposure is sudden and severe. Normally, a single large exposure is involved. – Examples are carbon monoxide, hydrofluoric acid, hydrogen cyanide and nicotine • Chronic toxin: prolonged or repeated exposures of a duration measured in days, months or years. Symptoms may not be immediately apparent. – Examples of chemicals of high chronic toxicity include dimethylmercury, nickel carbonyl, benzo-a-pyrene, Nnitrosodiethylamine, and other human carcinogens or substances with high carcinogenic potency in animals 23
Carcinogens, mutagens and teratogens • One of the most significant chronic risks associated with chemicals is their potential to cause cell mutation and proliferation. – Carcinogen: chemicals that can increase the incidence of cancer in the body – Mutagen: chemicals that cause mutations in DNA that lead to hereditary genetic defects in a fetus • There are two other general classifications that you should be aware of: – Teratogen: chemicals that induce non-hereditary malformations of a fetus – Sensitizer: chemicals that no reaction in a person during initial exposures, but further exposures will cause an allergic response to the chemical 24

Routes of Entry and Allowable Exposure Limits • There are four main routes by which hazardous chemicals enter the body: – Inhalation: Absorption through the respiratory tract. Most important in terms of severity. – Skin absorption. – Ingestion: Absorption through the digestive tract. Can occur through eating or smoking with contaminated hands or in contaminated work areas. – Injection. Can occur by accidental needle stick or puncture of skin with a sharp object. • Most exposure standards, Threshold Limit Values (TLVs) and Permissible Exposure Limits(PELs), are based on the inhalation route of exposure. expressed in terms of either parts per million (ppm) or milligrams per cubic meter (mg/m 3) concentration in air. • Other measures of chemical exposure: • Lethal dose or concentration for 50% of the exposed population (LD 50 or LC 50) expressed in mg contaminant per kg of body weight • Short term exposure limit (STEL or TLV-STEL) is the amount of a substance you can be exposed to for 15 minutes four times a day 25

5. Fire Safety 26

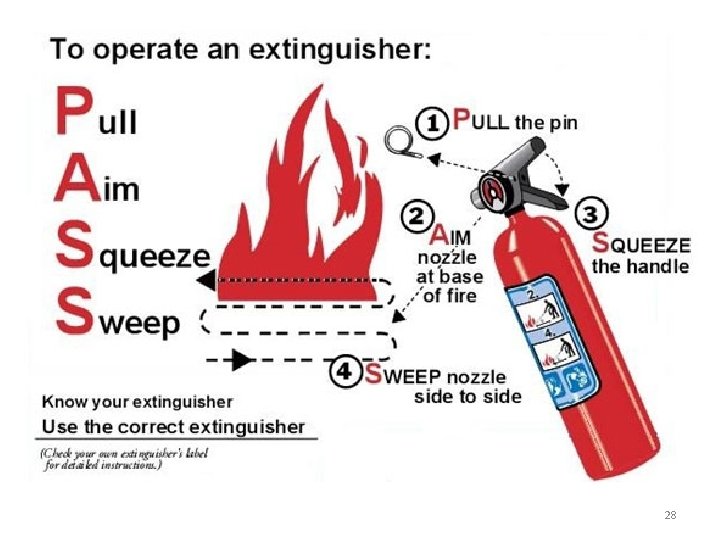
Fire Alarms & Extinguishers Where are the ones closest to you? 27
28

Types of Fire Extinguishers This is a special fire extinguisher for combustible metal fires. It is a type D fire extinguisher. You won’t need to use this unless you work in a research lab with combustible metals. Most of our fire extinguishers are ABC. It contains a dry powder to put out the kinds of fires we might encounter in the chemistry labs where we have class. 29

Student Reaction in a Fire Although we want you to be informed on the operation of a fire extinguisher, we do not expect you to use it. If a fire is ignited in your area, the proper STUDENT response is to: 1) Notify everyone in the room 2) If possible shutdown any reaction in progress by removing heat/energy source and/or pulling plug on power cord 3) Proceed to the nearest exit and pull the nearest fire alarm 4) Evacuate the building 5) Assemble in front of the library for a positive headcount 30

Flammables, combustibles, and potentially explosive materials • There are different ways of designating that a chemical is a fire risk: • Flashpoint - minimum temperature at which a liquid gives off a vapor in sufficient concentration to ignite in the presence of an ignition source • Combustible liquid - Any liquid having a flashpoint at or above 37. 8 o. C (100 F) but below 93. 3 o. C (100 F) • Flammable liquid - any liquid having a flashpoint below 37. 8 o. C • Autoignition temperature - the lowest temperature at which it will spontaneously ignite in a normal atmosphere without an external source of ignition, such as a flame or spark • Explosive - A chemical that causes a sudden, almost instantaneous release of pressure, gas, and heat temperature. 31
Bad things can happen!! ü You need to be aware of what you are doing, and the risks of what you are doing. ü You also need to be aware of what others in the same lab room are doing, and the risks of what they are doing. 32

Working with flames • Flames are never allowed when flammable gases or liquids are in use near by. • Always alert others before lighting a flame. • Never leave a flame unattended under any circumstances. • Do NOT heat reactions with flames!!! • Turn off the natural gas at the valve when you are finished with your work. • In the synthesis lab, Bunsen burners are used to either (1) to pull TLC spotting tubes; rarely (2) to dry glassware or very rarely (3) to conduct flame or combustion tests. • Drying glassware with a Bunsen burner can only be done if Closed valve is ALL flammable materials are taken out of the hood first. Perpendicular to hose • Placing glassware in the drying oven overnight is the preferred method to dry glassware, so plan ahead!! 33

Guides On Procedures • • • For some processes or procedures there will be Departmentally approved (and EHS approved) Standard Operating Procedures (SOP) that must be studied and followed. For some processes or procedures there will be technical notes or experimental descriptions (these may or may not be formally sanctioned by the Department or EHS) that must be studied and followed. In all cases, if the Department or EHS mandate it, a formal SOP takes precedence over an instructor’s technical notes or experimental description. SOP’s are generated to address known hazards of an extreme nature (like handling tert-butyl lithium) or if there is a perceived need (by instructor, Department, or EHS) for an SOP. If you feel that an SOP is needed, but one is not in place, please let BOTH your instructor (or lab supervisor) and Department chair know about your concern. Generating an SOP is not always fast, but in some cases, it may be necessary. www. cchem. berkeley. edu/rsgrp/SOPs/Organolithium. doc www. baylor. edu/content/services/document. php/203287. doc 34

Working with Syringes • There are many types of syringes – Plastic or glass – Leur lock or non-Leur Lock 35

Caution if using non-Leur-Lock Caution if using Plastic • The needle and syringe can be easily separated if not “locked” in place with the Leur-Lock system. • Use of non-Leur-Lock connections requires TWO hands, one on the connection, one on the plunger/syringe • Plastic syringes dissolve in many organic solvents • Plastic syringes and non-Leur Lock syringes should NEVER be used to transfer pyrophoric materials 36

UCLA Lab Fire: December 29, 2008 Sheri Sangji was using this plastic syringe to transfer tert-butyllithium. This was not the correct procedure, because this compound is well-known to ignite if it is comes in contact with air. The syringe plunger dropped out of the syringe and the reagent ignited. Sheri died January 16, 2009 of severe burns. She was wearing nitrile gloves but no lab coat. The students assisting her did not remember to put her under the safety shower. 37

Lessons from UCLA accident Lessons: Know the proper procedures for transferring dangerous reagents. Wear your lab coat at all times in the lab. Know where safety shower and other emergency equipment is – you may need to be the one who needs to be ready to act when your lab mate is unable to help himself/herself. 38

Do not stab yourself!!! • Use only ONE hand if recapping a syringe needle. • Use needles only once if they come in contact with reagents or solvents. • Put all used needles for disposal into an approved “Sharpes” container. 39

Working with Silica Gel • Inhalation of fine silica gel particles can lead to a disabling, irreversible, horrible disease called “silicosis”. • Sand at the beach is silica gel, but its particle size is large compared to silica gel used for chromatography. • Treat silica gel used for chromatography as an extremely hazardous substance. • Don’t eat it of course, but remember it is VERY unsafe to inhale. 40

Working with a Vacuum Line • Vacuum lines are often used to strip off trace, otherwise tightly adsorbed solvent molecules. • Vacuum lines are often used to purge (remove) and then charge (refill) the atmosphere over a reaction to remove unwanted oxygen or atmospheric moisture. • When put under a vacuum, glassware is stressed. • A star crack in the bottom of a flask subjected to a strong vacuum could cause the flask to implode. • Implosions can cause as much damage or harm as an explosion. 41

Working with An Inert Gas line • Make sure there is a pressure relief pathway for gas to escape BEFORE turning on the gas supply. • Literally trace the flow of gas from the outlet to the atmosphere, through a bubbler, with a finger BEFORE turning on the gas supply. • Use the minimum positive pressure necessary to keep atmospheric gases from entering the system. • Do not use a gas line if the low pressure regulator exceeds 20 psi. 42

Working with a Rotary Evaporator: Let’s go RV’ing. • • • Get a tare weight of final RBF before removing the solvent. Save time by RV’ing in stages: start with large RBF work down to small RBF. Never fill any RBF over half full before RV’ing. Clean up after AND before each use for a single concentration. The glassware is thick walled and expensive. The rotovap glassware should be either coated in plastic or wrapped with electrical tape. Make sure the solvent trap is empty after AND before using the rotovap. Put collected solvent stripped off by rotovap into waste container. Never apply a vacuum to a heated solution. Apply the vacuum, then warm it up. Spin it fast enough to avoid bumping, but use bump bulb all the same. The order for turning on the rotovap (attaching RBF, applying vacuum, closing stop cock, lowering into water bath) is the opposite of the order for turning it off (raise out of water bath, open stop cock, turn off vacuum, remove RBF). 43

Working with Compressed Air • It can really jet out: show some caution. • It can really make a lot of noise: show some consideration. • It might have grease in it: do not use on “pure” compounds. • It might not actually work: the compressor can be broken. 44

7. Disposal Procedures 45

Broken Glassware • Always check your glassware and discard any with chips, breaks, or obvious flaws. • Throw away broken glassware into special glass waste containers, NOT the trash. YES NO 46

Glove Recycling Contaminated Gloves with visible signs of chemical exposure or those used with hazardous substances should be collected in solid waste or biohazard containers. Uncontaminated Gloves that have no visible sign of chemical exposure or residue can be placed in the glove recycling container. If there’s any questions or the boxes need attention, please contact Dr. Katherine Mullaugh. Email: mullaughkm@cofc. edu Phone: 843. 953. 6587 47

Waste Disposal • Waste containers are provided for chemical waste generated in laboratories • Some things can go down the sink, some can’t. Always check with your instructor. • Care must be used to avoid mixing incompatible chemicals such as – Acids with Bases – Oxidizers and Flammables – Water reactive and aqueous solutions – Cyanides and acids 48

RECORD ALL WASTE DEPOSITS For each addition to the aqueous, organic or solid waste container you MUST record Month/Date; Your Initials, ID of waste, and amount of waste. USE THE WASTE STREAM LIST FOR THE APPROPRIATE CONTAINER: e. g. : Et. OAc with trace hexane impure sodium benzoate 2: 1 CH 2 Cl 2: hexane ~200 m. L ~5 g ~55 m. L 49

Handling Waste in Organic Labs • Organic liquids like CH 2 Cl 2 (aka methylene chloride, dichloromethane) & acetone, & TBME & liquid reagents PUT IN ORGANIC (HALOGENATED) LIQUID WASTE CONTAINER IN WASTE HOOD • Aqueous – neutral (not basic or acidic) containing trace organics PUT IN AQUEOUS WASTE CONTAINER IN WASTE HOOD • Aqueous – neutral (not basic or acidic) containing NONTOXIC salts with no trace organics CAN GO IN PUBLIC SEWAR, DOWN THE DRAIN (Use the “Would I want to swim it rule? ”. Yes? Then put it down the drain. NO? Then put it in the aqueous waste container. ) • Solid chemical – old products, left over starting materials, includes organic and inorganic PUT IN SOLID WASTE CONTAINER IN WASTE HOOD • Solid, non toxic waste (paper towels, notebook pages) PUT IN TRASH ONLY IF SAFE TO TOUCH WITH BARE HANDS 50

Think First, Dispose Second Ü PAPER, WITHOUT CHEMICAL X X RESIDUE CLEAN BROKEN GLASS CHEMICAL WASTE X X PAPER, WITHOUT CHEMICAL RESIDUE CLEAN BROKEN GLASS Ü CHEMICAL WASTE X PAPER, WITHOUT CHEMICAL RESIDUE Ü CLEAN BROKEN GLASS X CHEMICAL WASTE 51

8. How to be a good lab citizen 52

SEVEN must-have habits for lab-work 1. 2. 3. 4. 5. 6. 7. Be prepared before walking into the lab. Think about the how and why before doing anything. Begin with a clean, neat work area; make it so. Minimize clutter; store book bags, equipment, etc. Have instructions, pen and notebook available. Return materials and equipment to proper places. Make it clean and neat and orderly before leaving. 53
Chemical storage • • • Flammables/combustibles Acids Bases Oxidizers Nonreactive (e. g. , brine) 54

Storing Acids and Bases 1. 2. 3. 4. 5. 6. Mineral Acids (like HCl, H 3 PO 4, H 2 SO 4) Organic Acids (like ptsa) Aqueous Inorganic Bases (like Na. OH) Non-aqueous Inorganic Bases (like Na. H, Na) Lithiated Organic Bases (like n-Bu. Li, LDA) Nitric Acid (Strong Oxidizer)

The color of the bottle cap has a meaning. Cap color corresponds to concentrated solution of: • • • Red: Nitric Acid Blue: Hydrochloric Acid Yellow: Yellow Sulfuric Acid Brown: Acetic Acid White: Phosphoric Acid Also one base: • Green: Ammonium Hydroxide For solutions try to use: • clear, black, orange, or purple caps.

Chemical Spills • Notify your instructor and your neighbors if you spill chemicals on the floor or bench. • Don’t try to clean it up yourself. Your instructor may need to use a specially designed chemical spill kit. 57

8. Procedures and Practices 58

Students must report any injuries, big or small. • Report all injuries to the instructor. We will not call emergency services unless the instructor determines it is a serious injury. • An incident report will be filled out whether it is small or serious. 59

Injury procedure, continued • First Aid kits are available in the lab with band aids and other items for treating small cuts and burns. • Campus public safety can be reached at 35609 for non-emergencies. • If it is a serious injury, call 911 for emergencies. • The Live. Safe app can also be used to report emergencies and non-emergencies. 60

Process safety When performing an experiment always consider the following: • – Is the material flammable, explosive, corrosive, or reactive? – Is the material toxic, and if so, how exposure to the material occur – What kind of personal protective equipment or ventilation is needed to protect myself? – Will the process generate other toxic compounds, or could it result in a fire, explosion, etc. ? – Are storage facilities appropriate for the type of materials used? Can incompatible materials be properly segregated? – What possible accidents can occur and what steps can be taken to minimize the likelihood and impact of an accident? – What are the proper procedures for disposal of the chemical(s)? As an example of process safety consider distillation: 61

Glassware Set Ups • Have apparatus inspected by instructor before using it • Have apparatus elevated off bench top so heat can be removed quickly if needed • Have a clamp around neck of flask so if heat source is removed, apparatus is still supported • Make sure water flow goes uphill, and cooling water ends up going unimpeded down a drain. • If leaving water on overnight, the hose connections MUST be secured into place with wire or strip pulls. • Perform inside hood, behind safety shield, with shield between your face and the apparatus. 62
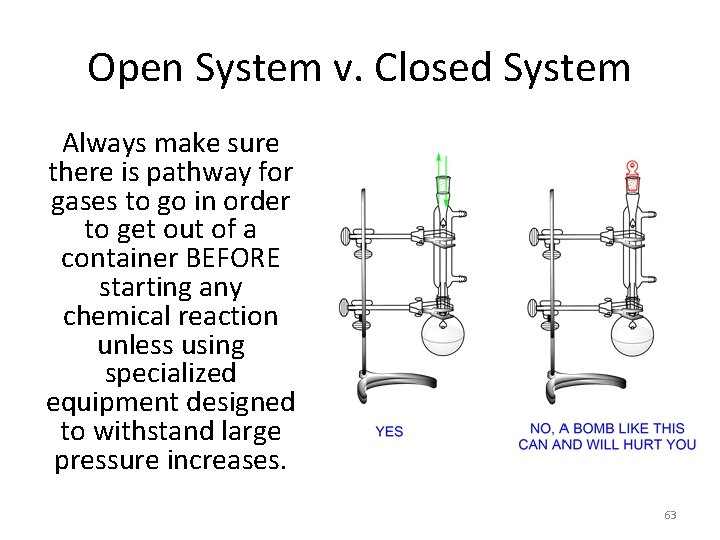
Open System v. Closed System Always make sure there is pathway for gases to go in order to get out of a container BEFORE starting any chemical reaction unless using specialized equipment designed to withstand large pressure increases. 63

OSHA FACT SHEET Laboratory Safety Chemical Hygiene Plan (CHP) OSHA’s Occupational Exposure to Hazardous Chemicals in Laboratories standard (29 CFR 1910. 1450), referred to as the Laboratory standard, specifies the mandatory requirements of a Chemical Hygiene Plan (CHP) to protect laboratory workers from harm due to hazardous chemicals. The CHP is a written program stating the policies, procedures and responsibilities that protect workers (at Cof. C “workers” includes faculty, students and staff) from the health hazards associated with the hazardous chemicals used in that particular workplace. 64

Cof. C - CHP Who wrote the Cof. C CHP? • Director of Environmental Health & Safety (with input from faculty & staff) Where can you find the Cof. C CHP? • In the lab • On the lab web site or OAKS page • On Departmental Web Site 65

GHS • GHS: the Global Harmonization System • GHS is being incorporated by OSHA into the Hazards Communication Standard (HAZCOM) that ensures people who handle chemicals are properly trained • New symbols for labels with universal usage are being developed: 66
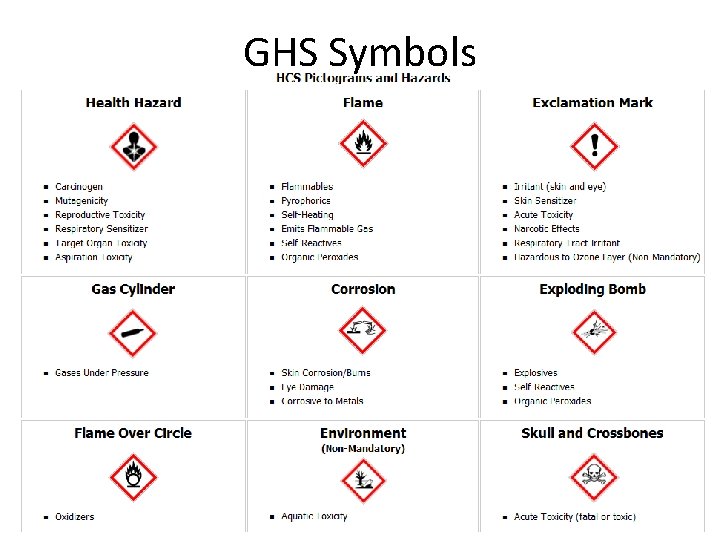
GHS Symbols

Report any concerns • If you have any safety concerns about the lab you are working in or the people working around you, you can contact: 1. To your instructor 2. Dr. Marcello Forconi– Head of the departmental safety committee 3. Dr. Pamela Riggs-Gelasco – Department Chair for Chemistry and Biochemistry 4. Dr. Jim Deavor, Associate Dean of the School of Science and Mathematics. 68
- Slides: 68