Safe Injection Practices Speaker Sue Dill Calloway RN



































































































































- Slides: 191

Safe Injection Practices

Speaker § Sue Dill Calloway RN, Esq. CPHRM § AD, BA, BSN, MSN, JD § President § Patient Safety and Healthcare Consulting § 5447 Fawnbrook Lane § Dublin, Ohio 43017 § sdill 1@columbus. rr. com § 614 791 -1468 2

3

4

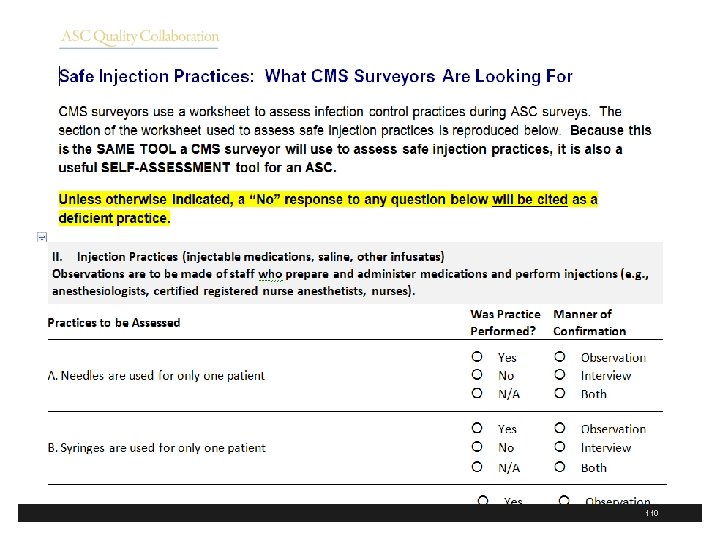
5

6

7

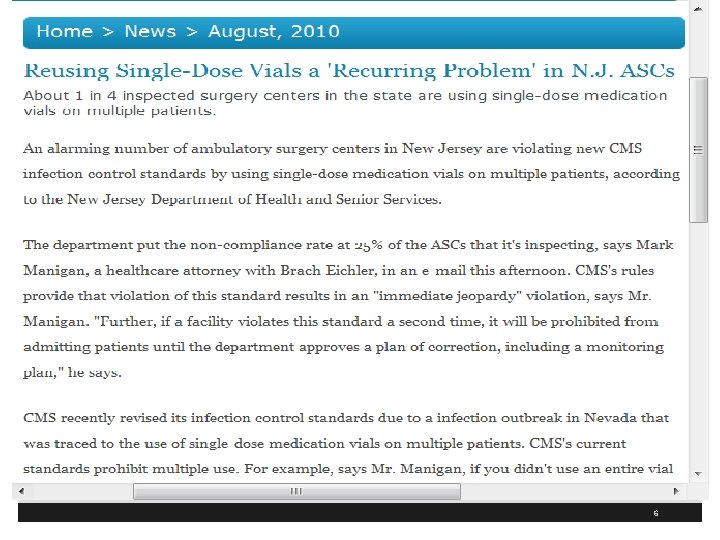
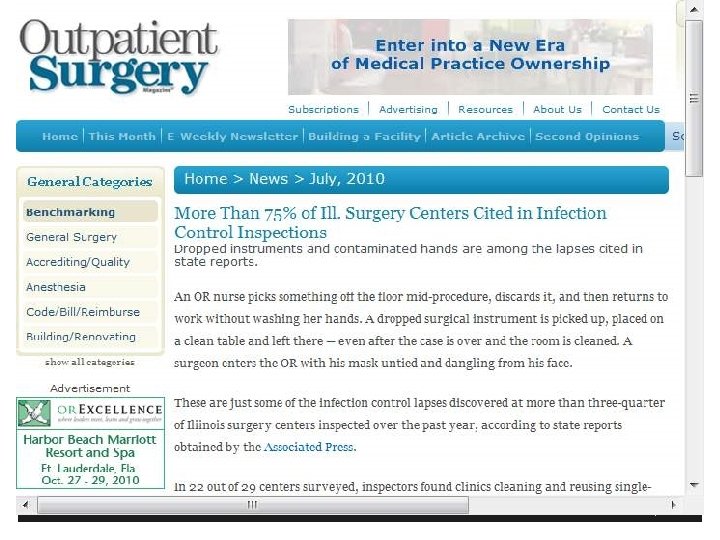
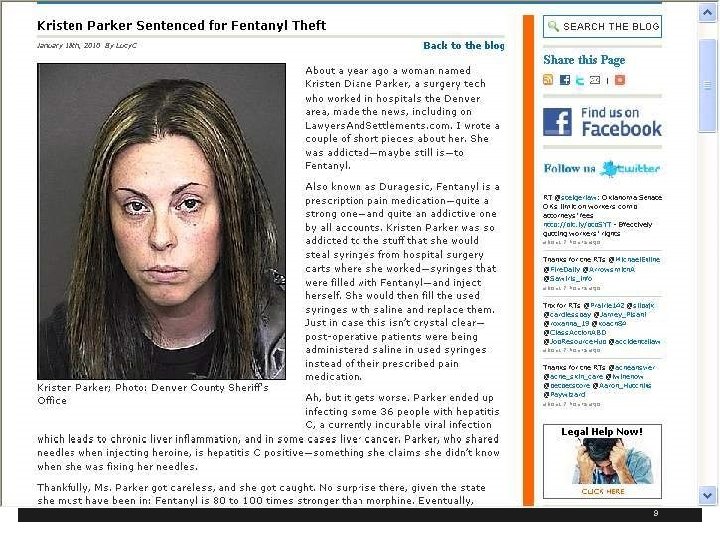
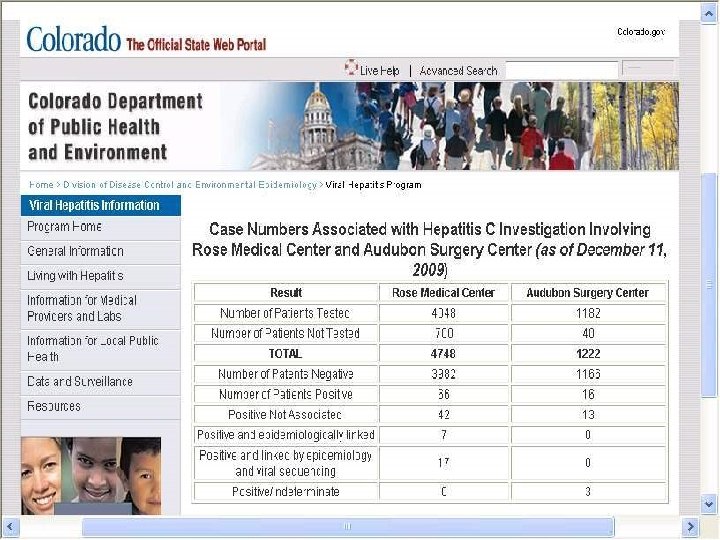
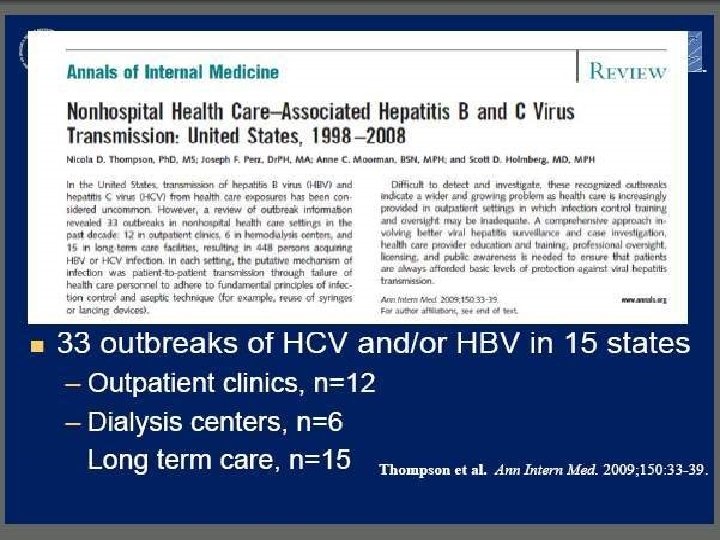
Identify Risks for Transmitting Infections § Hospital and ASC in Colorado where surgery tech with Hepatitis C infection steals Fentanyl and replaces it with used syringes of saline infecting 17 patients as of December 11, 2009 and 5, 970 patients tested (total 36 for 3 facilities) § Kristen Diane Parker in 2010 gets 30 years for drug theft and needle swap scheme § Worked at Denver’s Rose Medical Center and Colorado Springs’ Audubon Surgery Center § 1 www. krdo. com/Global/link. asp? L=399119 8

9

10

Infection Control § The CDC says there are 1. 7 million healthcare infection (HAI) in America every year § There are 99, 000 deaths in American hospitals every year § Leadership need to make sure there is adequate staffing and resources to prevent and manage infections § Healthcare-Associated Infections (HAIs) are one of the top ten leading causes of death in the US 1 § 1 www. cdc. gov/ncidod/dhqp/hai. html 11

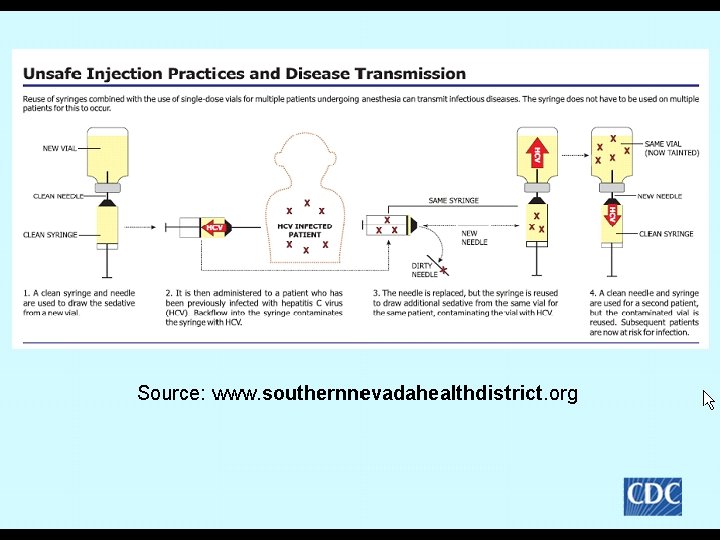
Infection Control § There have been more than 35 outbreaks of viral hepatitis in the past 10 years because of unsafe injection practices § This has resulted in the exposure of over 100, 000 individuals to HBV and 500 patients to HCV § This includes inappropriate care of maintenance of finger stick devices and glucometers § Includes syringe reuse, contaminations of vials or IV bags and failure of safe injection practices § Source: APIC position paper: Safe injection, infusion, and medication vial practices in health care 12

Infection Control Back to Basics § It is important to get back to basics in infection control 1 § Education and training is imperative to learn each person’s role in preventing infections § What practices and constant reminders do you use to remind staff during patient care encounters? § New needle and syringe for every injection § Single dose saline syringes § 1 http: //www. jcrinc. com/infection-prevention-back-to-basics/ 13

What is Injection Safety or Safe Injection Practices? § The CDC says it is a set of measures taken to perform injections in an optimally safe manner for patients, healthcare personnel, and others § A safe injection does not harm the recipient, does not expose the provider to any avoidable risks and does not result in waste that is dangerous for the community § Injection safety includes practices intended to prevent transmission of infectious diseases between one patient and another, or between a patient and healthcare provider, and also to prevent harms such as needle stick injuries 14

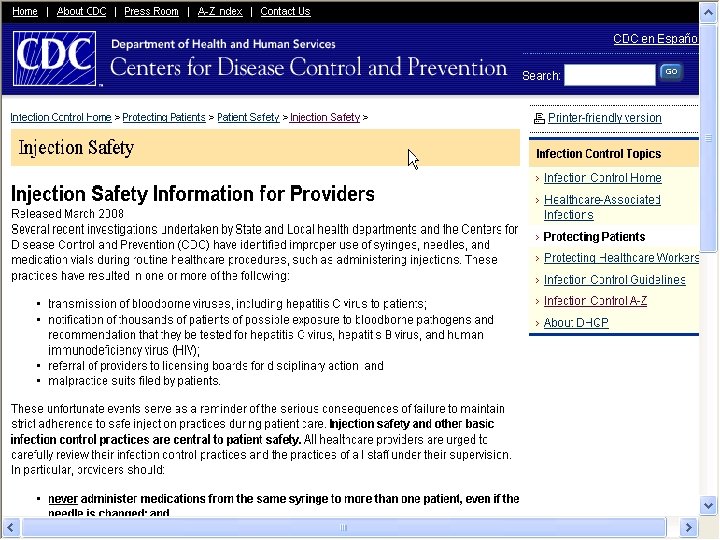
CDC Injection Safety Website § The CDC has an injection safety website § Contains information for providers § Injection Safety FAQs § Safe Injection Practices to Prevent Transmissions of Infections to Patients § Section from Guidelines for the Isolation Precautions to Prevent Transmission and more § www. cdc. gov/ncidod/dhqp/injectionsafety. html 15

16

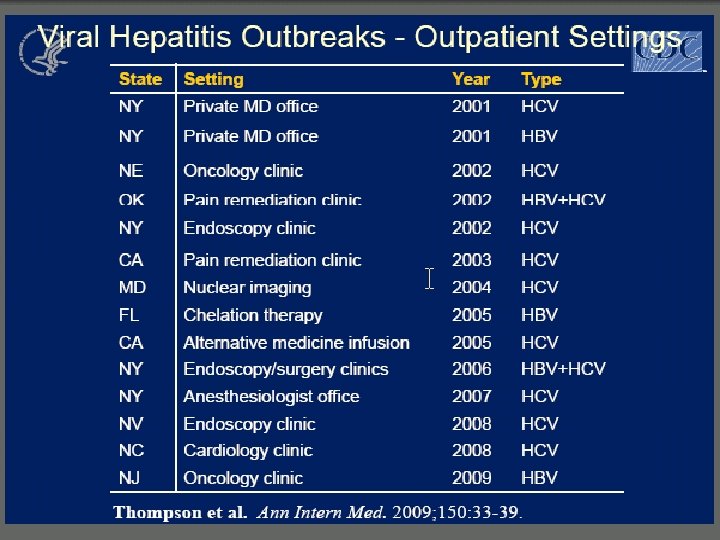
CDC Guidelines § CDC has a publication called 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings § Has a section on Safe Injection Practices (III. A. 1. b. and starts on page 68) § Discusses four large outbreaks of HBV and HCV among patients in ambulatory facilities § Identified a need to define and reinforce safe injection practices www. cdc. gov/hicpac/pdf/isolation/Isolation 2007. pdf 17

18

19

20

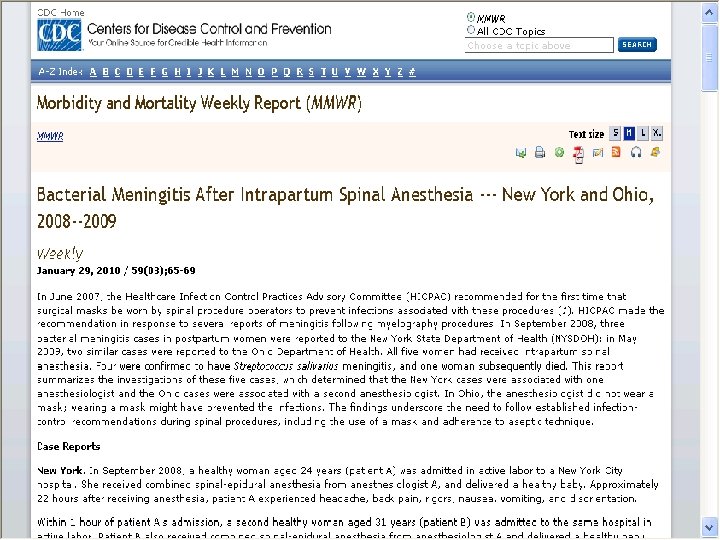
Lumbar Puncture Procedures § CDC investigated 8 cases of post-myleography meningitis § Streptococcus species from oropharngeal flora § None of the physicians wore a mask § Droplets of oral flora indicated § Lead to CDC recommendations of 2007 § Later related to not wearing a mask when anesthesiologists put in epidural lines for pain relief on women in labor 21

CDC Guidelines § Recently, five cases where anesthesiologist inserts epidural line in OB patients without wearing a mask § January 29, 2010 CDC MMWR at www. cdc. gov/mmwr/preview/mmwrhtml/mm 5903 a 1. htm § CDC made recommendation in June 2007 after several reports of meningitis after myelograms § Bacterial meningitis in postpartum women and Ohio woman dies May 2009 § Streptococcus salivarius meningitis (bacteria that is part of normal mouth flora) 22

Wear Mask When Inserting Epidural/Spinal § Hospital in NY – Enhanced hand hygiene – Maintenance of sterile fields – Full gown, gloves, and mask – No visitors when epidural put in § CDC has only identified 179 cases of post spinal (including lumbar punctures) world wide from 1952 to 2005 23

24

CDC Guidelines § CDC identified four outbreaks in § Pain clinic § Endoscopy clinic § Hematology/oncology clinic § Will discuss major findings later 25

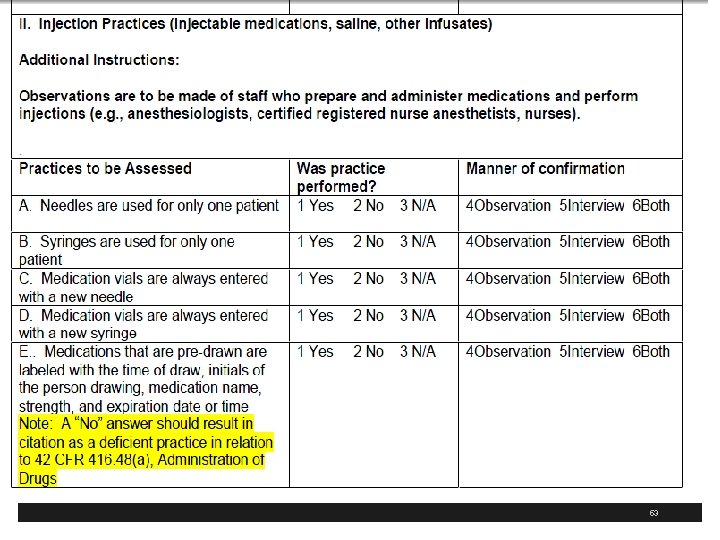
CDC Guidelines § Primary breaches § Reinsertion of used needles into multidose vials § Used 500 cc bag of saline to irrigate IVs of multiple patients § Use of single needle or syringe to administer IV medications to multiple patients § Preparing medications in same work space where syringes are dismantled § Remember OSHA Bloodborne Pathogen standard (sharps containers at the bedside) 26

27

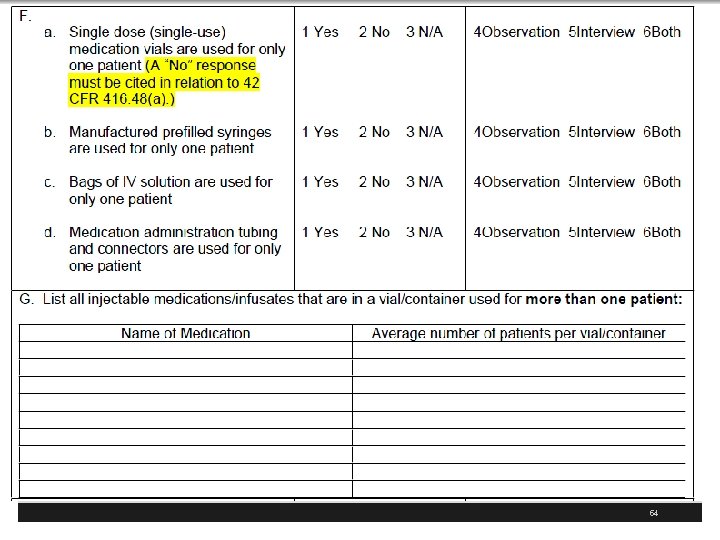
What to Do? § Use only single dose vials and not multidose vials when available § This includes the use of saline single dose flushes § Single use of a disposal needle and syringe for each injection § Prevent contamination of injection equipment and medication 28

What to Do? § Wear masks when inserting epidural or spinals § Discard used syringe intact in appropriate sharps container § Make sure sharps container in each patient room § Do not administer medications from single dose vials to multiple patients or combine left over contents for later use 29

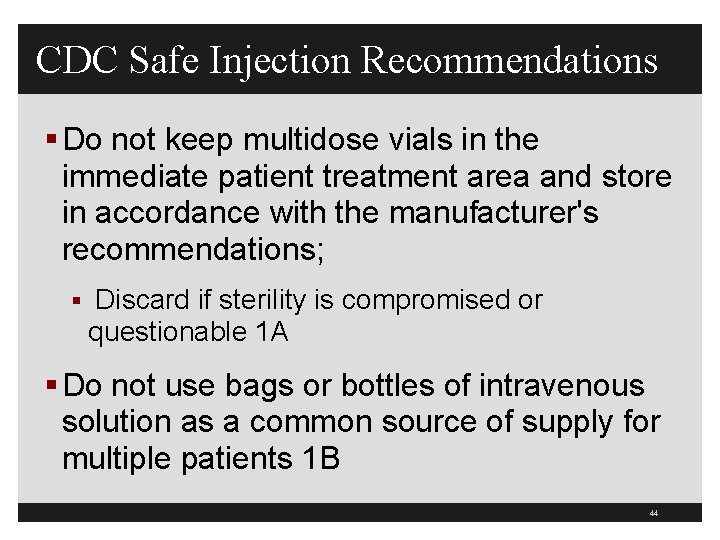
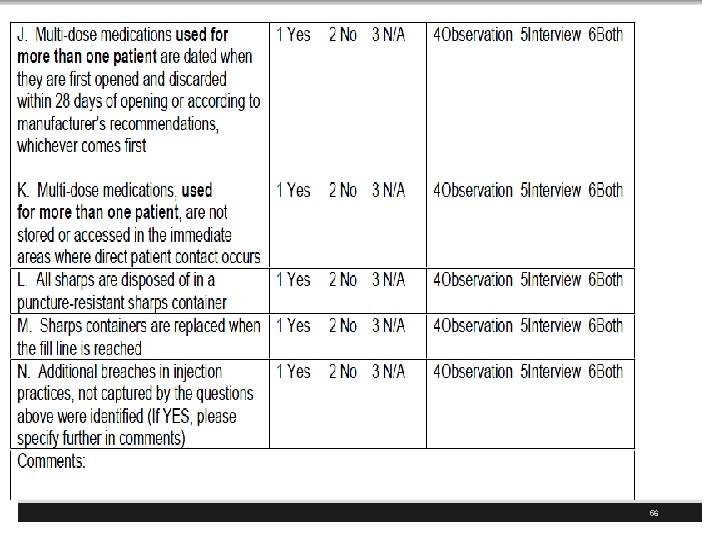
What to Do? § If multiple-dose vials are used, restrict them to a centralized medication area or for single patient use § Never re-enter a vial with a needle or syringe used on one patient if that vial will be used to withdraw medication for another patient § Store vials in accordance with manufacturer’s recommendations and discard if sterility is compromised § Mark date on multi-dose vial 30

What to Do? § Do not use bags or bottles of intravenous solution as a common source of supply for multiple patients § Follow the CDC 10 recommendations § Maintaining clean, uncluttered, and functionally separate areas for product preparation to minimize the possibility of contamination § CMS Hospital Co. P requirement, tag 501 § TJC 2010 MM. 05. 01. 07 § Clean top with Bleach wipe after each use 31

A Scary Study § The CDC says a survey of US Healthcare found that 1% to 3% reused the same syringe and/or the same needle on multiple patients § This is what lead to the Nevada patients being exposed to HIV, HCV, and HCB § 40, 000 patients were notified who has anesthesia injections from March 2004 to January 11, 2008 and 115 patients infected with HCV § Clinic reused syringes in colonoscopies and other gastrointestinal procedures 32

33

34

Please Ask Me § The Ask Me Program and the Nevada Medical Association posts information on their website § The Nevada State Health Division has encouraged patients to ask several questions prior to a surgical procedure http: //health. nv. gov/docs/030308 Press. Release. pdf § Can you assure me that I am safe in your facility from the transmission of communicable diseases? 35

Please Ask Me Program § How does the staff at this facility conduct sterilization of diagnostic equipment after each patient use? § Are single or multiple dose vials used at the facility? Are label instructions followed specifically? § Are syringes and needles disposed of after each use? § Has your facility ever received a complaint of the spread of an infectious disease to another patient as a result of staff practices? 36

CDC Injections Safety for Providers § The CDC also issues Injection Safety for Providers § Issued March 2008 at http: //www. cdc. gov/ncidod/dhqp/ps_provider. Info. html § Notes several investigations leading to transmission of Hepatitis C to patients § Thousands of patients notified to be test for HVB, HCV, and HIV § Referral of providers to the licensing boards for disciplinary actions § Malpractice suits filed by patients 37

38

CDC 10 Recommendations § The CDC has a page on Injection Safety that contains the excerps from the Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings § Summarizes their 10 recommendations § Available at http: //www. cdc. gov/ncidod/dhqp/injection. Safety. Pr actices. html 39

40

CDC Safe Injection Recommendations § Use aseptic technique to avoid contamination of sterile injection equipment. Category 1 A § Do not administer medications from a syringe to multiple patients, even if the needle or cannula on the syringe is changed. § Needles, cannula and syringes are sterile, single-use items; they should not be reused for another patient nor to access a medication or solution that might be used for a subsequent patient. 1 A 41

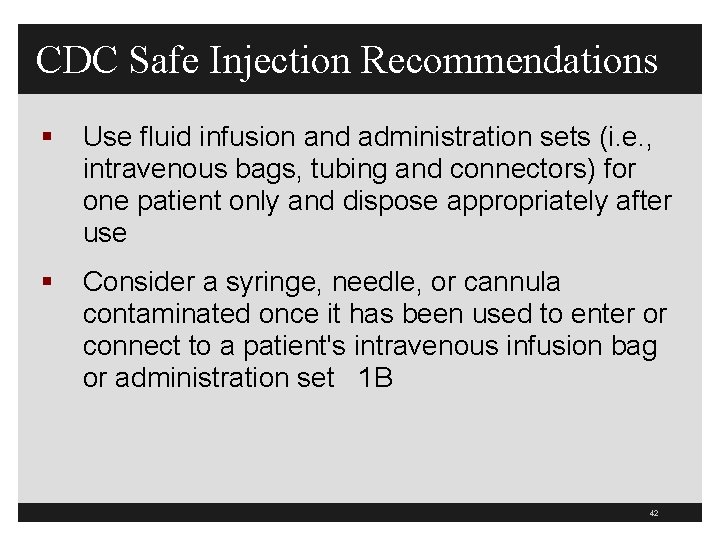
CDC Safe Injection Recommendations § Use fluid infusion and administration sets (i. e. , intravenous bags, tubing and connectors) for one patient only and dispose appropriately after use § Consider a syringe, needle, or cannula contaminated once it has been used to enter or connect to a patient's intravenous infusion bag or administration set 1 B 42

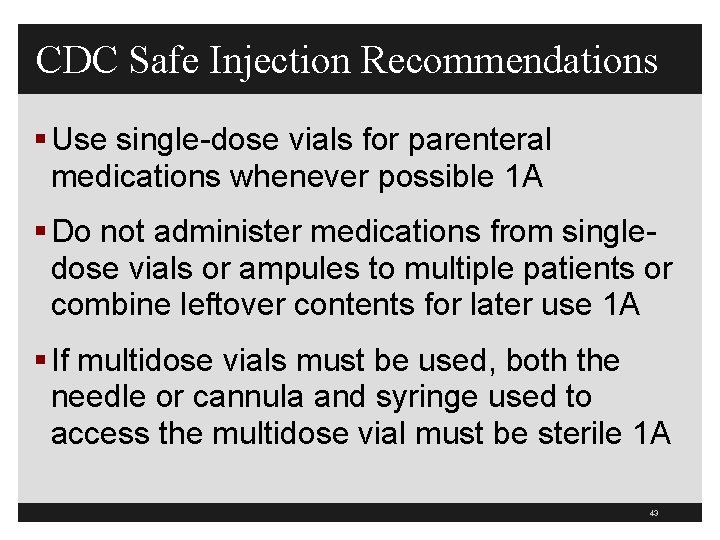
CDC Safe Injection Recommendations § Use single-dose vials for parenteral medications whenever possible 1 A § Do not administer medications from singledose vials or ampules to multiple patients or combine leftover contents for later use 1 A § If multidose vials must be used, both the needle or cannula and syringe used to access the multidose vial must be sterile 1 A 43

CDC Safe Injection Recommendations § Do not keep multidose vials in the immediate patient treatment area and store in accordance with the manufacturer's recommendations; § Discard if sterility is compromised or questionable 1 A § Do not use bags or bottles of intravenous solution as a common source of supply for multiple patients 1 B 44

CDC Safe Injection Recommendations § Wear a mask when placing a catheter or injecting material into the spinal canal or subdural space § Example, during myelograms, lumbar puncture and spinal or epidural anesthesia. 1 B § Worker safety; Adhere to federal (OSHA) and state requirements for protection of healthcare personnel from exposure to blood borne pathogens 1 B 45

CDC has Injection Safety FAQs for Providers § CDC has another resources with frequently asked questions § What is injection safety? § Incorrect practices identified in IV medications for chemotherapy, cosmetic procedures, and alternative medicine therapies § Available at http: //www. cdc. gov/ncidod/dhqp/injection. Safety. FA Qs. html 46

47

CDC has Injection Safety FAQs for Providers § Also puts patients at risk for bacterial and fungal infections beside HIV and Hepatitis § Single dose vials do not contain a preservative to prevent bacterial growth so safe practices necessary to prevent bacterial and viral contamination § Proper hand hygiene before handling medications § Make sure contaminated things are not placed near medication preparation area 48

CDC has Injection Safety FAQs for Providers § Single use parenteral medication should be administered to one patient only § Pre-filled medication syringes should never be used on more than one patient § A needed or other device should never be left inserted into a medication vial septum for multiple uses § This provides a direct route for microorganisms to enter the vial and contaminate the fluid 49

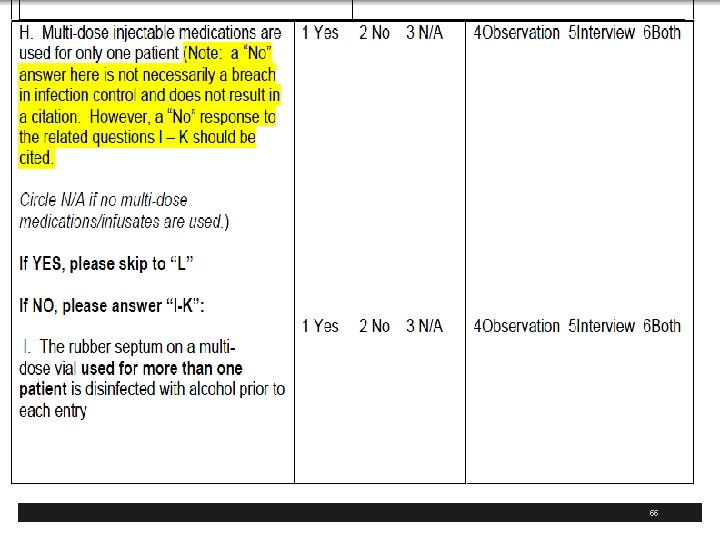
CDC has Injection Safety FAQs for Providers § Multi-dose Vials § The safest thing to do is restrict each medication vial to a single patient, even if it's a multi-dose vial § Proper aseptic technique should always be followed § If multi-dose medication vials must be used for more than one patient, the vial should only be accessed with a new sterile syringe and needle § It is also preferred that these medications not be prepared in the immediate patient care area 50

CDC has Injection Safety FAQs for Providers § To help ensure that staff understand adhere to safe injection practices, we recommend the following: § Designate someone to provide ongoing oversight for infection control issues § Develop written infection control policies § Provide training § Conduct performance improvement assessments 51

52

53

54

55

56

57

USP 797 § USP published a revision to the USP general Chapter of 797 § These standards apply to pharmacy compounded sterile preparation § This includes injections, nasal inhalations, suspensions for wound irrigations, eye drops etc. § Applies to the pharmacy setting as well as to all persons who prepare medications that are administered § And it applies to all healthcare centers 58

USP 797 § This chapter includes standards for preparing, labeling, and discarding prepared medications § Pharmacies compound sterile preparations under laminar flow hoods with stringent air quality and ventilation to maintain the sterility of the drug (ISO class 5 setting) § If prepare outside the pharmacy then environment has particulates and microorganisms increasing the potential for contaminating the vial, IV solution or syringes § Need to wash hands before preparing medication outside the pharmacy 59

USP 797 § Want to prepare IVs and piggybacks in the pharmacy when at all possible § Breathing over the sterile needle and vial stopper can create the potential for microbial contamination § USP exempts preparation outside the pharmacy for immediate use § 1 hour limit from completing preparation and this includes spiking an IV bag § Cost of medication disposal can be daunting if case not started within one hour which is why should consider pharmacy preparing under ISO class 5 environment 60

USP 797 § This way the drugs used for surgery are prepared by properly trained, cleansed, and garbed personnel to prolong the usability of the immediate use compounded sterile drugs (CSD) § These can be stored for 48 hours § Another option is to located a manufacturers injectable product (prepackaged syringe) that is discarded according to manufacturer expiration date § APIC supports preparing parenteral medication as close as possible to the time of administration 61

USP 797 APIC Recommendations § Make sure only trained staff are preparing medications § Need to prepared in a clean dry workspace that is free of clutter and obvious contamination sources like water, sinks § Medications should be stored in a manner to limit the risk of tampering § Should verify the competency of those preparing medications and monitor compliance with aseptic technique § 28 day discard date on multidose vials even though CDC says manufacturers recommendations 62

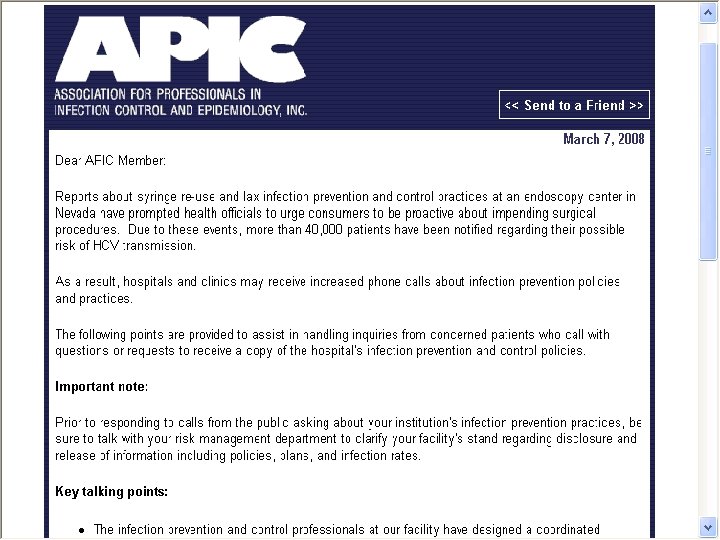
APIC Recommendations § APIC issues recommendations and key talking points for hospitals and healthcare facilities § http: //apic. informz. net/apic/archives/archive_27223 5. html § The infection preventionist at our facility has designed a coordinated infection control program § This is protect everyone coming in to our facility § Our program implements evidenced based practices from leading authorities including the CDC 63

APIC Recommendations § Cleanse the access diaphragm of vials using friction and a sterile 70% isopropyl alcohol, ethyl alcohol, iodophor, or other approved antiseptic swab § Allow the diaphragm to dry before inserting any device into the vial § Never store or transport vials in clothing or pockets. § Discard single-dose vials after use § Never use them again for another patient § Use multi-dose medication vials for a single patient whenever possible 64

APIC Recommendations § Never leave a needle, cannula, or spike device inserted into a medication vial rubber stopper because it leaves the vial vulnerable to contamination § even if it has a 1 -way valve § Use a new syringe and a new needle for each entry into a vial or IV bag § Utilize sharps safety devices whenever possible § Dispose of used needles/syringes at the point of use in an approved sharps container 65

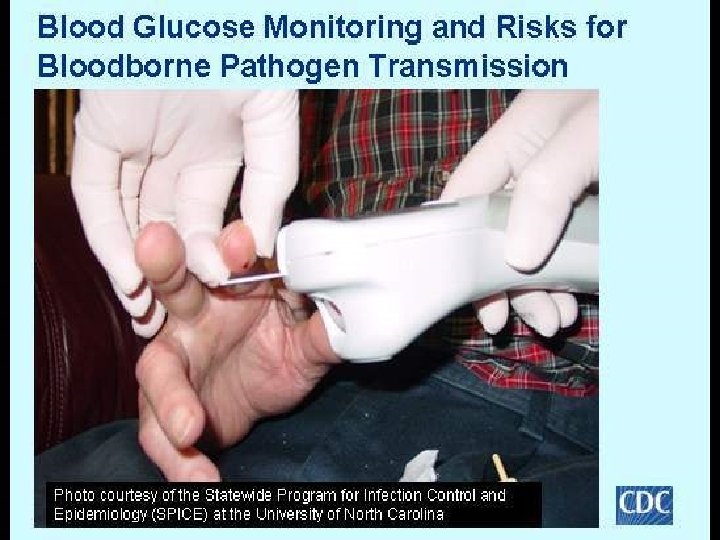
Blood Glucose Monitoring Devices APIC 66

67

68

APIC Key Talking Points § This program includes § Rigorous hand hygiene practices § Monitoring the cleaning disinfection, and sterilization of equipment and instruments § An Exposure Control Plan that serves to minimize bloodborne pathogens such as HIV, Hepatitis B and C by patients and staff § As part of this program there are measures to prevent the re-use of items designed to be used only once such as needles and syringes 69

70

A Patient Safety Threat-Syringe Reuse § CDC published a fact sheet called “A Patient Safety Threat- Syringe Reuse” § It was published for patients who had received a letter stating they could be at risk due to syringe reuse § Discusses the dangers of the reuse of syringes § Discusses that multidose vial be assigned to a single patient to reduce the risk of disease transmission 71

72

73

Anesthesia Delivery § Nevada clinics started with Lidocaine 1 cc and Propofol 9 ccs in one syringe § Clean needle and syringe initially § If patient needed more used clean needle but used old syringe § If medication left in the single dose Propofol vial used to sedate the next patient 74

Anesthesia Delivery § Propofol is single dose medication and preservative free § Bought 20 -50 cc vials but only used 10 -15 cc per patient § Clinic had not had full inspection by state surveyors in 7 years § Identified a number of infection control problems with ASC § CMS has new freestanding ASC CMC Cf. Cs May 18, 2009 and revised December 30, 2009 75

Never Event: Unsafe Injection Practices § The CDC has a website entitled “ A Never Event: Unsafe Practices” § Has a power point presentation and an audio presentation § Available at www. cdc. gov/ncidod/dhqp/COCA_Unsafe_I njection_Practices. html 76

77

78

Hematology Oncology Clinic § Has an outbreak of HCV among outpatients 3 -00 to 7 -01 § Reported to Nebraska Health Department § 99 patients with clinic acquired HCV after having chemotherapy § All were genotype 3 a which is uncommon in the US § Related to catheter flushing § Source: Macedo de Oliveira et al. , Annals of Internal Medicine, 2005, 142: 898 -902 79

Hematology Oncology Clinic § Nurse drew blood from the IV catheter § Then she reused the same syringe to flush the catheter with saline § She did use a new syringe for each patient § However, she used solution from same 500 cc bag for multiple patients § Oncologist and RN license revoked § Never use an IV solution bag to flush the solution for more than patient 80

81

82

83

Other Cases § Patient in US gets malaria from saline flush § Emerging Infectious Diseases, Vol 11, No. 7, July 2005 § Oklahoma Pain Clinic where anesthesiologist filled syringe with sedation medication to treat up to 24 patients and injected via hep lock § 71 patients with HCV and 31 with HBV § 25 million dollar settlement § Source: Comstock et al. ICHE, 2004, 25: 576 -583 84

Other Cases § 19 patients get HCV in New York in 2001 from contamination of multi-dose anesthesia vials § CDC MMWR September 26, 2003, Vol 52, No 38 § NY City private physician office with 38 patients with HBV § Associated with injections of vitamins and steroids § Gave 2 or 3 in one syringe § Source: Samandari et al. ICHE 2005 26 (9); 745 -50 85

Bacterial Outbreak Due to Unsafe Needle § 7 patients get serratia marcescens from spinal injections in a pain clinic § Source: Cohen Al et al. Clin J Pain 2008; 24(5): 374 -380 § Several other studies where patients got infection from joint and soft tissue injections § Got staph aureus § In 2003 and 2009 86

Dialysis Facilities § CDC issues MMWR report April 2008 § Dialysis units must follow CDC guidelines to receive Medicare payments for outpatient services § Recent outbreaks of HCV and other bacterial infections § From reentry into single dose medication vials to more than one patient § CDC recommends to use single dose vials 87

88

Dialysis Facilities § If multi-dose then should be assigned to one person § Should be prepared in a clean area separate from potentially contaminated surfaces § Medications should be prepared in clean area removed from the patient treatment area because surfaces are subjected to frequent blood contamination 89

Injections Safety and Recent Outbreaks § The CDC website has a slide presentation called “Injection Safety & Recent Outbreaks” § From APIC North Carolina October 5, 2009 § Has 48 slides § Available at http: //www. cdc. gov/ncidod/dhqp/injectionsafet y. html 90

91

92

93

94

WHO Injection Safety § The World Health Organization also has resources on injection safety § Recently had 10 th annual meeting of the Safe Injection Global Network (SIGN) § Has revised injection safety assessment tool § 73 pages document § http: //www. who. int/injection_safety/en/ 95

96

97

WHO Safe Injection Tool 98

99

WHO § Also has a 51 pages document § Covers the 2008 conference that was held in Moscow § Additional information about the Safe Injection Global Network (SIGN) § Includes a report of the SIGN 100

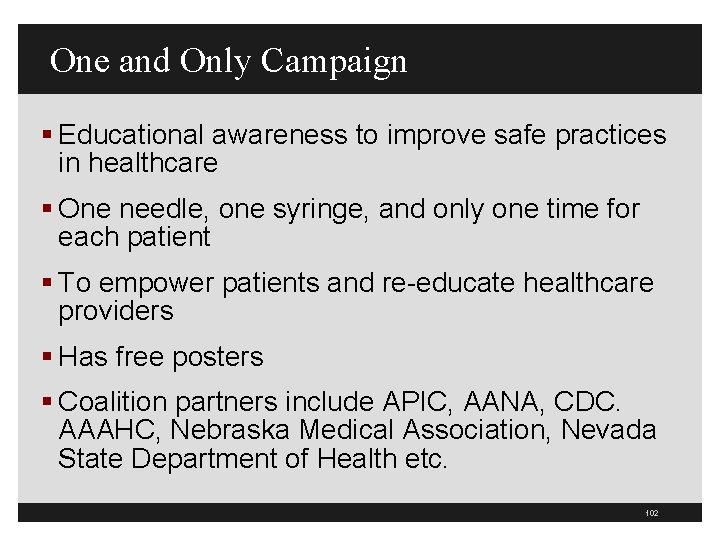
101

One and Only Campaign § Educational awareness to improve safe practices in healthcare § One needle, one syringe, and only one time for each patient § To empower patients and re-educate healthcare providers § Has free posters § Coalition partners include APIC, AANA, CDC. AAAHC, Nebraska Medical Association, Nevada State Department of Health etc. 102

http: //oneandonlycampaign. org/ 103

104

105

106

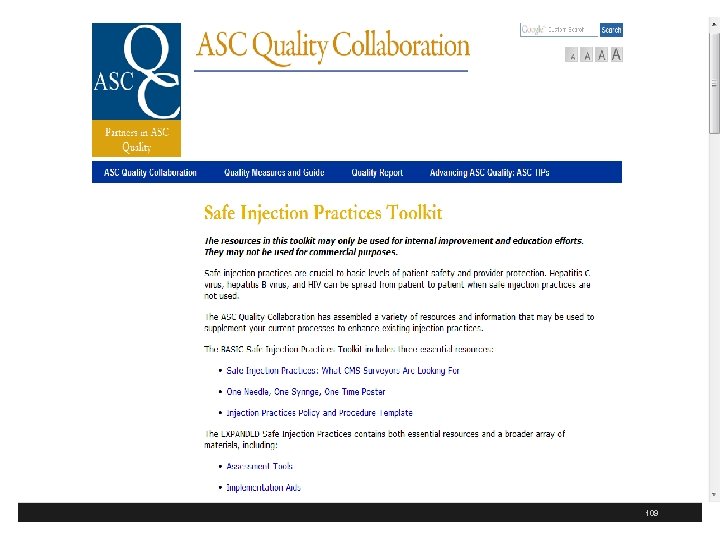
Advancing ASC Quality § ASC Quality Collaboration has ASC tool kit for infection prevention § Includes one on hand hygiene and safe injection practices § Includes a basic and expanded version of the toolkit § These are available at http: //www. ascquality. org/advancing_asc_quality. cfm 107

108

109

110

111

112

113

The End Questions § Sue Dill Calloway RN, Esq. CPHRM § AD, BA, BSN, MSN, JD § President § Patient Safety and Healthcare Consulting § 5447 Fawnbrook Lane § Dublin, Ohio 43017 § 614 791 -1468 § sdill 1@columbus. rr. com § Avoiding Needlestick Follows 114

Avoiding Needle Stick Injuries

Speaker § Sue Dill Calloway RN, Esq. CPHRM § AD, BA, BSN, MSN, JD § President § Patient Safety and Education § 5447 Fawnbrook Lane § Dublin, Ohio 43017 § 614 579 -1481 § sdill 1@columbus. rr. com 116

OSHA § Ten years after the Needlestick Safety and Prevention Act was signed into law § Which is part of the OSHA Bloodborne Pathogen Standard (29 CFR 1910. 1030) § OSHA announces a regulatory review of the law § Has this standard had a impact on healthcare worker safety? § Recent article says sharps in non-surgical setting has declined by about 32% 1 § 1 Jagger J, Berguer R, Phillips EK, et al. Increase in sharps injuries in surgical settings versus non-surgical settings after passage of national needlestick legislation. J Amer Col Surg 2010; 210: 496 -502 117

OSHA § Safely engineered devises have resulted in 74% decrease in injuries in phlebotomy § However, this is not true in the surgery operating room where adoption of blunt suture needles and other sharps safety measures have lagged § Sharps injury has increased from 1993 to 2006 by 6. 5% § This regulation remains the most frequent cited standard in OSHA inspections of hospitals 118

OSHA § Inspectors were most likely to cite for failing to have an adequate exposure control plan or failing to update the plan to reflect changes in technology § The standard requires employers to review their exposure control plans annually § Hospitals also were cited for failing to provide safety-engineered devices § Or failing to document that employees had been offered the hepatitis B vaccine § The same types of violations are being seen by ASCs 119

www. osha. gov/SLTC/bloodbornepathogens/index. html 120

Needlestick Safety and Prevention Act § The Occupational Exposure to Bloodborne Pathogen Standard was first published in 1991 § Passed because of concerns to healthcare workers of things such as HIV, hepatitis B and C who were exposed to blood or other potentially infectious materials § saliva, blood, semen, cerebrospinal fluid, amniotic, synovial, pleural, pericardial, peritoneal etc § Employer needed an exposure control plan on details on employee protection measures § Engineering controls included safer medical devices, such as needleless devices, shielded needle devices and plastic capillary tubes 121

Needlestick Safety and Prevention Act § Despite these advances with non-needle devises needlestick and sharps injuries continued § OSHA said there were nearly 600, 000 percutaneous injuries involving sharps so Congress passed the Needlestick Safety and Prevention Act which became effective April 18, 2001 (passed November 6, 2000) § Still requires employers to adopt engineering and work practice controls that would eliminate or minimize employee exposure from hazards associated with bloodborne pathogens 122

Needlestick Safety and Prevention Act § Need to pull out your exposure control plan every year § Need to do an annual review § Need to update to reflect changes in technology that help to eliminate or reduce exposure to bloodborne pathogens § Take into consideration new safer devices designed to reduce needlestick injuries § Document consideration and use of appropriate safer devices 123

Sample Model Plans from OSHA www. osha. gov/Publications/osha 3186. html 124

125

126

Needlestick Safety and Prevention Act § List employees involved and describe how input was requested or present minutes of meetings § Employers need to get input from employees responsible for direct patient care (non management such as nurses) on evaluation, identification and selection of effective and safer devices § Employees selected should include those exposure in different areas like peds, geriatrics, nuclear medicine etc. 127

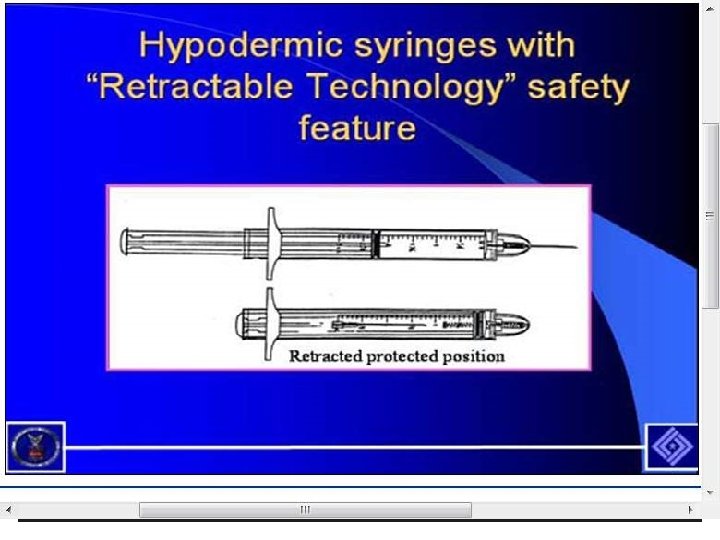
Needlestick Safety and Prevention Act § Engineering controls include things that isolate or remove a hazard from the workplace § Such as sharp disposal containers and self-sheathing needles § Sharps with engineered sharps injury protection (SESIP) includes nonneedle sharps or needle devices with safety features including § Syringes with a sliding sheath that shields the attached needle after use § Needles that retract into a syringe after use § Shielded or retracting catheters § IV delivery systems that use a catheter port with a needle housed in a protective covering 128

Needlestick Safety and Prevention Act § Needless systems include IV medication using a port with non needle connections or jet injection system that deliver liquid medicine under the skin or through a muscle § Employers must keep a Sharps Injury Log for the recording of percutaneous injuries from contaminated sharps § Remember that sharps containers must be easily accessible to employees and located as close as feasible to the immediate area where sharps are used 129

www. cdc. gov/niosh/sharps 1. html 130

131

132

133

134

www. osha. gov/SLTC/bloodbornepathogens/index. html 135

Sharps Safety § Have a policy and procedure on sharps safety § Include safety measures to prevent injury during perioperative care § Use double gloving, blunt suture needles for fascial closing and neutral zones, when appropriate, to avoid hand to hand passage of sharps § Include references position statements in P&P and where these are located 1 1 www. cspsteam. org/sharpssafety. html / 136

Blunt Tip Suture Needles § Surgical personnel are at risk of bloodborne injuries from sharp surgical instruments § OSHA has document on the “Use of Blunt-Tip Suture Needles to Decrease Percutaneous Injuries to Surgical Personnel: Safety and Health Information Bulletin” 1 § Sharp tip suture needles are the leading source of percutaneous injuries to surgical personnel causing 51 to 77% of these incidents § 1 http: //www. cdc. gov/niosh/docs/2008 -101/ 137

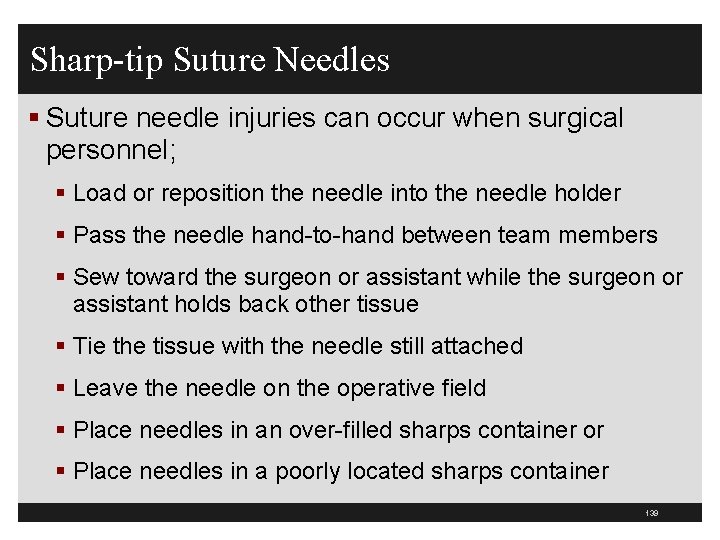
138

Sharp-tip Suture Needles § Suture needle injuries can occur when surgical personnel; § Load or reposition the needle into the needle holder § Pass the needle hand-to-hand between team members § Sew toward the surgeon or assistant while the surgeon or assistant holds back other tissue § Tie the tissue with the needle still attached § Leave the needle on the operative field § Place needles in an over-filled sharps container or § Place needles in a poorly located sharps container 139

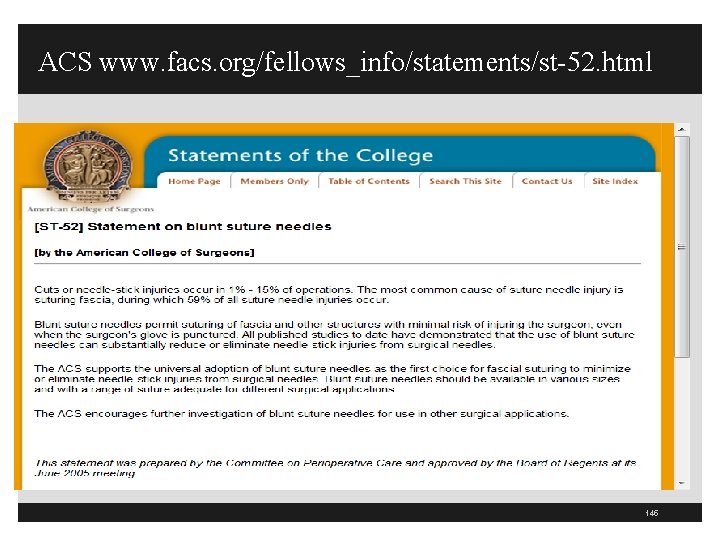
National Associations Blunt Tip Suture § American College of Surgeons ACS) recommends in 2005 the universal adoption of blunt-tip suture needles for suturing fascia § Also encourages further investigation of their appropriate use in other surgical applications § AORN endorsed this ASC statement in support of blunt-tip suture needles where effective and clinically appropriate § Other organizations endorse such as ASA, ASPAN, AANA, American Association of Surgical PAs, and the Association of Surgical Technologists 140

141

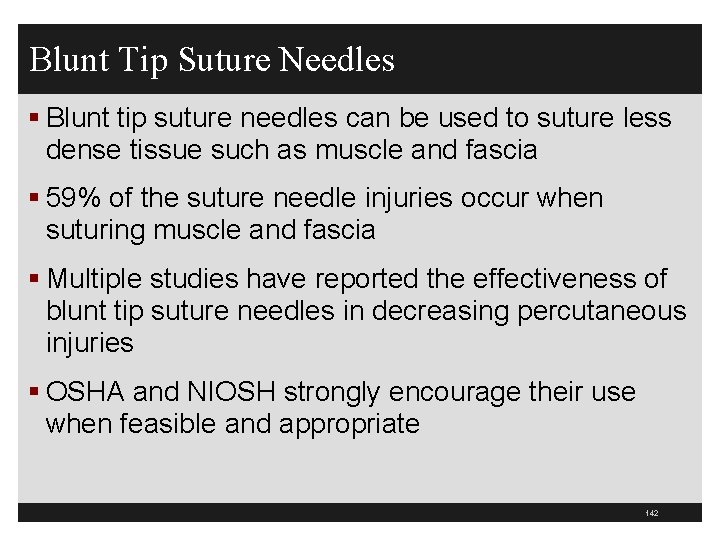
Blunt Tip Suture Needles § Blunt tip suture needles can be used to suture less dense tissue such as muscle and fascia § 59% of the suture needle injuries occur when suturing muscle and fascia § Multiple studies have reported the effectiveness of blunt tip suture needles in decreasing percutaneous injuries § OSHA and NIOSH strongly encourage their use when feasible and appropriate 142

/ 143

AORN 2010 Page 697 Perioperative Standards and Recommended Practices 144

ACS www. facs. org/fellows_info/statements/st-52. html 145

146

147

148

www. cdc. gov/sharpssafety/ 149

Free Workbook from the CDC 150

151

International Sharps Injury Prevention Society www. isips. org/ 152

www. jointcommission. org/Sentinel. Events/Sentinel. Event. A lert/sea_22. htm 153

http: //www. tdict. org/ 154

155

156

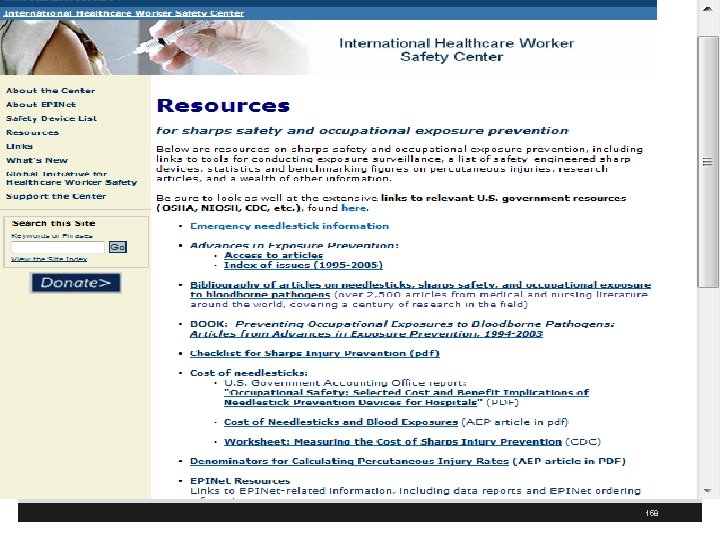
www. healthsystem. virginia. edu/internet/epinet// 157

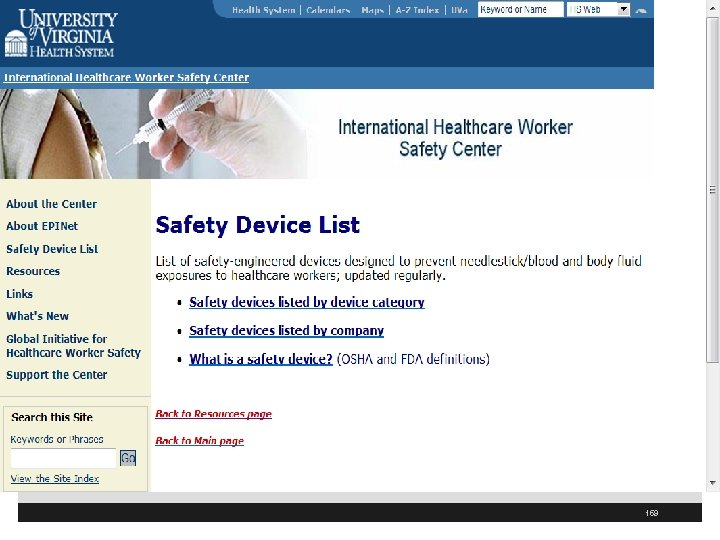
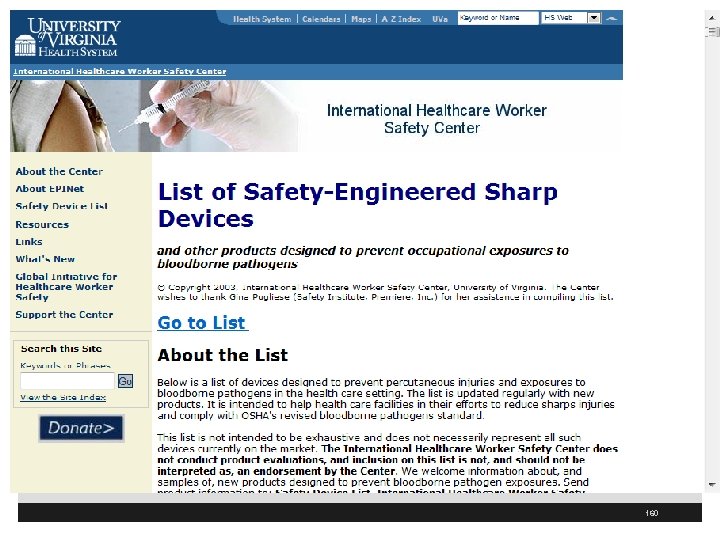
158

159

160

http: //nursingworld. org/Main. Menu. Categories/Occupationaland. Envir onmental/occupationalhealth/Safe. Needles. aspx 161

162

163

www. cdc. gov/niosh/topics/bbp/ndl-law. html 164

www. facs. org/about/committees/cpc/preventingsharpsinjuries. pdf 165

166

167

168

169

170

Resources § Jagger J, Bentley M, Tereskerz P. A study of patterns and prevention of blood exposure in OR personnel. AORN J. 1998; 67(5): 979 -81, 983 -4, 986 -7 § Berguer R, Heller PJ. Preventing sharps injuries in the operating room. J Am Coll Surg. 2004; 199(3): 462 -7 § Makary MA, Al-Attar A, Holzmueller CG, Sexton JB, Syin D, Gilson MM, Sulkowski MS, Pronovost PJ. Needlestick injuries among surgeons in training. N Engl J Med. 2007 Jun 28; 356(26): 2693 -9 171

Resources § Davis MS. Advanced Precautions for Today's OR: The Operating Room Professional's Handbook for the Prevention of Sharps Injuries and Bloodborne Exposures, 1 st ed. Atlanta; Sweinbinder; 1999. § American College of Surgeons (ACS). Statement on blunt suture needles. Bull Am Coll Surg. 2005 Nov; 90(11): 24. Available from http: //www. facs. org/fellows_info/statements/st 52. html 172

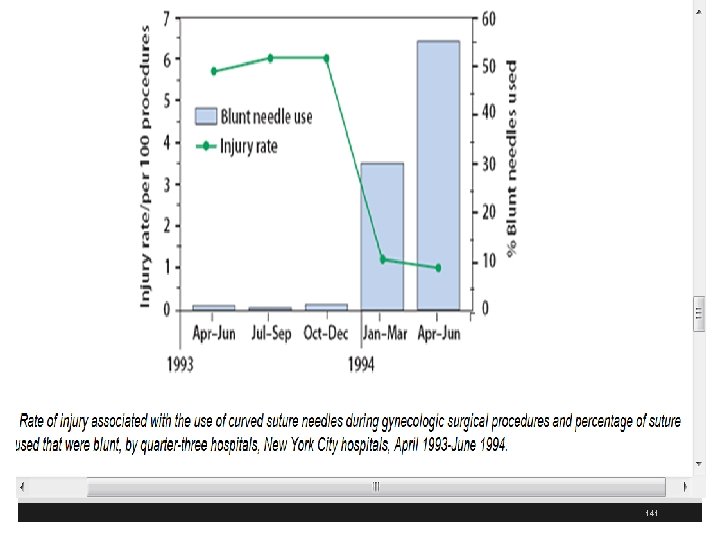
Resources § Association of Perioperative Registered Nurses (AORN). AORN Guidance Statement: Sharps Injury Prevention in the Perioperative Setting. In: 2005 Standards, Recommended Practices, and Guidelines. 2005; 199 -204. § Available from www. aorn. org/about/positions/pdf/SECTI-2 esharpssafety. pdf § Centers for Disease Control and Prevention (CDC). Evaluation of blunt suture needles in preventing percutaneous injuries among health-care workers during gynecologic surgical procedures-New York City, March 1993 -June 1994. MMWR Morb Mortal Wkly Rep. 1997; 46(2): 259. § http: //www. cdc. gov/ mmwr/preview/mmwrhtml/00045660. htm 173

Resources § CFR (Code of Federal regulations). Title 29 Part 1910, OSHA. Washington, DC: U. S. Government Printing Office, Office of the Federal Register § Dauleh MI, Irving AD, Townell NH. Needle prick injury to the surgeon-do we need sharp needles? J R Coll Surg Edinb. 1994; 39(5): 310 -1. § Jagger J, Berguer R, Phillips EK, et al. Increase in sharps injuries in surgical settings versus nonsurgical settings after passage of national needlestick legislation. J Amer Col Surg 2010; 210: 496 -502 174

Resources § Davis MS. Advanced Precautions for Today’s O. R. In: The Operating Room Professional’s Handbook for the Prevention of Sharps Injuries and Bloodborne Pathogen Exposures. Atlanta, GA: Sweinbinder Publications LLC; 2001. § Aarnio P, Laine T. Glove perforation rate in vascular surgery—A comparison between single and double gloving. Vasa. 2001; 30(2): 122 -124. § Berguer R, Heller PJ. Strategies for preventing sharps injuries in the operating room. Surg Clin North Am. 2005; 85(6): 1288 -305, xiii. 175

Resources § Caillot JL, Cote C, Abidi H, Fabry J. Electronic evaluation of the value of double gloving. Br J Surg. 1999; 86(11): 1387 -1390. § Dauleh MI, Irving AD, Townell NH. Needle prick injury to the surgeon—Do we need sharp needles? J R Coll Surg Edinb. 1994; 39(5): 310 -311. § Eggleston MK Jr, Wax JR, Philput C, et al. Use of surgical pass trays to reduce intraoperative glove perforations. J Matern Fetal Med. 1997; 6(4): 245247. 176

Resources § Evaluation of blunt suture needles in preventing percutaneous injuries among health-care workers during gynecologic surgical procedures—New York City, March 1993–June 1994. MMWR Morb Mortal Wkly Rep. 1997; 46(2): 25 -29. § Gerberding JL, Littell C, Tarkington A, et al. Risk of exposure of surgical personnel to patients’ blood during surgery at San Francisco General Hospital. N Engl J Med. 1990; 322(25): 1788 -1793. 177

Resources § Hartley JE, Ahmed S, Milkins R, et al. Randomized trial of blunt-tipped versus cutting needles to reduce glove puncture during mass closure of the abdomen. Br J Surg. 1996; 83(8): 1156 -1157 § Hollaus PH, Lax F, Janakiev D, et al. Glove perforation rate in open lung surgery. Eur J Cardiothorac Surg. 1999; 15(4): 461 -464. § Jagger J, Bentley M, Tereskerz P. A study of patterns and prevention of blood exposures in OR personnel. AORN J. 1998; 67(5): 979 -981, 983 -974, 986 -977. 178

Resources § Jensen SL. Double gloving—Electrical resistance and surgeons’ resistance. Lancet. 2000; 355(9203): 514 -515. § Laine T, Aarnio P. How often does glove perforation occur in surgery? Comparison between single gloves and a double-gloving system. Am J Surg. 2001; 181(6): 564 -566. § Mingoli A, Sapienza P, Sgarzini G, et al. Influence of blunt needles on surgical glove perforation and safety for the surgeon. Am J Surg. 1996; 172(5): 512516; 516 -517. 179

Resources § Montz FJ, Fowler JM, Farias-Eisner R, Nash TJ. Blunt needles in fascial closure. Surg Gynecol Obstet. 1991; 173(2): 147 -148. § Naver LP, Gottrup F. Incidence of glove perforations in gastrointestinal surgery and the protective effect of double gloves: A prospective, randomised controlled study. Eur J Surg. 2000; 166(4): 293 -295. § Quebbeman EJ, Telford GL, Hubbard S, et al. Risk of blood contamination and injury to operating room personnel. Ann Surg. 1991; 214(5): 614 -620. 180

Resources § Rice JJ, Mc. Cabe JP, Mc. Manus F. Needle stick injury. Reducing the risk. Int Orthop. 1996; 20(3): 132 -133. § Stringer B, Infante-Rivard C, Hanley JA. Effectiveness of the hands-free technique in reducing operating theatre injuries. Occup Environ Med. 2002; 59(10): 703 -707. § Tokars JI, Bell DM, Culver DH, et al. Percutaneous injuries during surgical procedures. JAMA. 1992; 267(21): 2899 -2904. 181

Resources § A recent CDC presentation on Unsafe Injection Practices, along with audio and a transcript of the presentation are available at: www. cdc. gov/ncidod/dhqp/COCA_Unsafe_ Injection_Practices. html § Re: infection control and injection practices www. cdc. gov/ncidod/dhqp/ps_provider. Info. html 182

Resources § Re: protecting patients from bloodborne pathogens in healthcare settings www. cdc. gov/ncidod/dhqp/bp_patient. html § Re: prevention of surgical site infections www. cdc. gov/ncidod/dhqp/gl_surgicalsite. html § Re: hand hygiene in healthcare facilities www. cdc. gov/handhygiene/ 183

Resources § Re: healthcare facility physical environment and infection control § www. cdc. gov/ncidod/dhqp/gl_environinfection. html § CDC’s home page for infection control provides links to additional information: § www. cdc. gov/ncidod/dhqp/index. html 184

Resources § Mast ST, Woolwine JD, Gerberding JL. Efficacy of gloves in reducing blood volumes transferred during simulated needlestick injury. J Infect Dis 1993; 168(6): 1589 -92. § Henry K, Campbell S, Collier P, Williams CO. Compliance with universal precautions and needle handling and disposal practices among emergency department staff at two community hospitals. Am J Infect Control 1994; 22(3): 129 -37. 185

Resources § Vaughn TE, Mc. Coy KD, Beekmann SE, Woolson RE, Torner JC, Doebbeling BN. Factors promoting consistent adherence to safe needle precautions among hospital workers. Infect Control Hosp Epidemiol 2004; 25(7): 548 -55. § Clarke SP, Rockett JL, Sloane DM, Aiken LH. Organizational climate, staffing, and safety equipment as predictors of needlestick injuries and near-misses in hospital nurses. Am J Infect Control 2002; 30(4): 207 -16. 186

Resources CDC Training on Hepatitis www. cdc. gov/hepatitis/Resources/Professionals/Training. Res ources. htm 187

Resources § Danzig LE, Short LJ, Collins K, et al. Bloodstream infections associated with a needleless intravenous infusion system in patients receiving home infusion therapy. JAMA 1995; 273(23): 1862 -4. 188

Resources § Patel PR, Larson AK, Castel AD, et al. Hepatitis C virus infections from a contaminated radiopharmaceutical used in myocardial perfusion studies. JAMA 2006; 296: 2005 --11. § CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCVrelated chronic disease. MMWR 1998; 47(No. RR 19). § Williams IT, Perz JF, Bell BP. Viral hepatitis transmission in ambulatory health care settings. Clin Infect Dis 2004; 38: 1592 --8. 189

Resources § Comstock RD, Mallonee S, Fox JL, et al. A large nosocomial outbreak of hepatitis C and hepatitis B among patients receiving pain remediation treatments. Infect Control Hosp Epidemiol 2004; 25: 576 --83. § Krause G, Trepka MJ, Whisenhunt RS, et al. Noscomial transmission of hepatitis C virus associated with the use of multidose saline vials. Infect Control Hosp Epidemiol 2003; 24: 122 --7. 190

The End Questions § Sue Dill Calloway RN, Esq. CPHRM § AD, BA, BSN, MSN, JD § Medical Legal consultant § 5447 Fawnbrook Lane § Dublin, Ohio 43017 § 614 791 -1468 191