Sac Anchoring Prosthesis A New Method for EVAR





- Slides: 19

Sac Anchoring Prosthesis: A New Method for EVAR Mark Wholey M. D. UPMC Shadyside Heart and Peripheral Vascular Institute, Director, The. Vascular Institute

DISCLOSURES Mark H. Wholey, MD Consulting Fees – Abbott Vascular, Medrad, Inc. , Cordis, a Johnson & Johnson company, Covidien, Access. Closure, Inc. Board Membership – Car. Mell Therapeutics

The Sac Anchoring AAA Prosthesis The Nellix System Confidential

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Consulting Fees/Honoraria Company Cordis, Boston Scientific, Abbott Vascular Medrad Inc. , Access Closure Major Stock Shareholder/Equity Boston Scientific, Nellix Royalty Income Covidian Inc. , Setagon

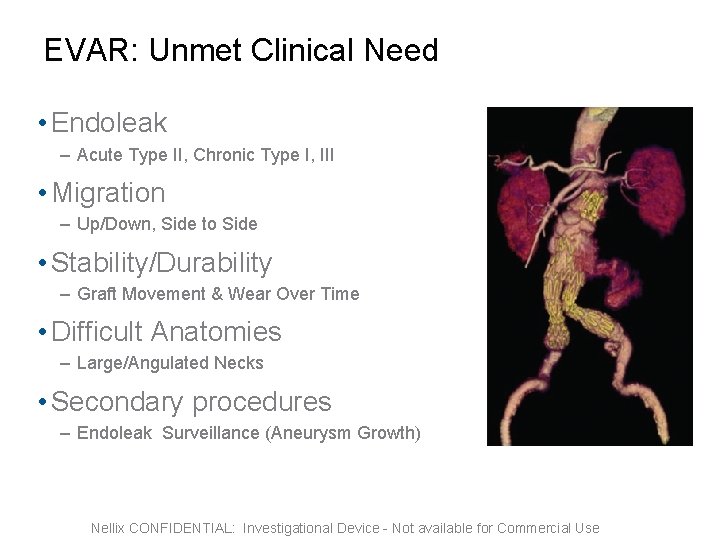
EVAR: Unmet Clinical Need • Endoleak – Acute Type II, Chronic Type I, III • Migration – Up/Down, Side to Side • Stability/Durability – Graft Movement & Wear Over Time • Difficult Anatomies – Large/Angulated Necks • Secondary procedures – Endoleak Surveillance (Aneurysm Growth) Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

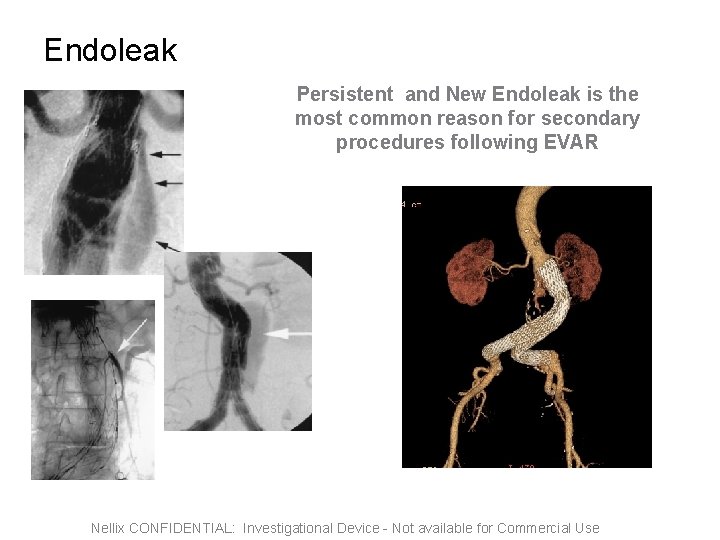
Endoleak Persistent and New Endoleak is the most common reason for secondary procedures following EVAR Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

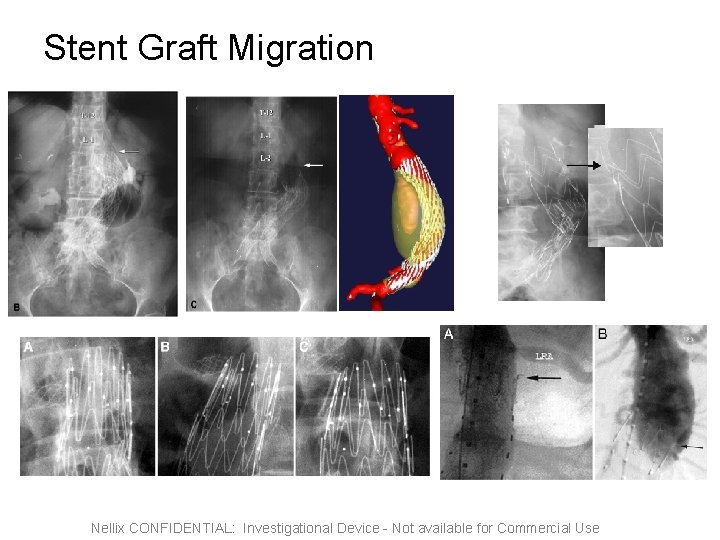
Stent Graft Migration Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

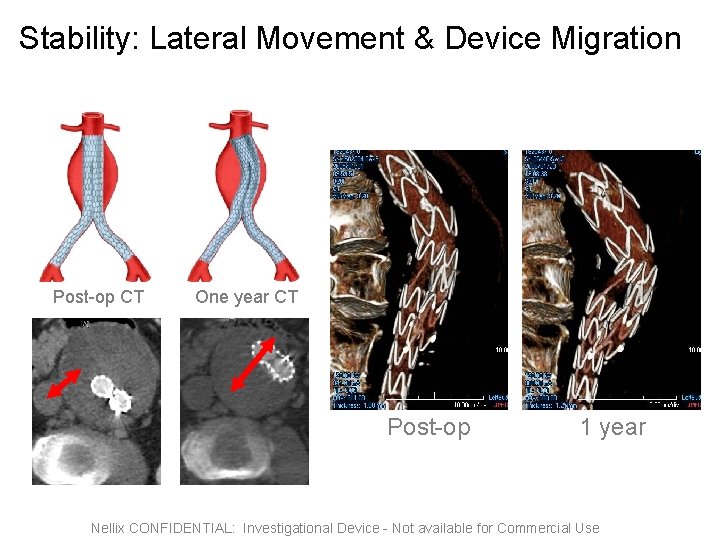
Stability: Lateral Movement & Device Migration Post-op CT One year CT Post-op 1 year Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

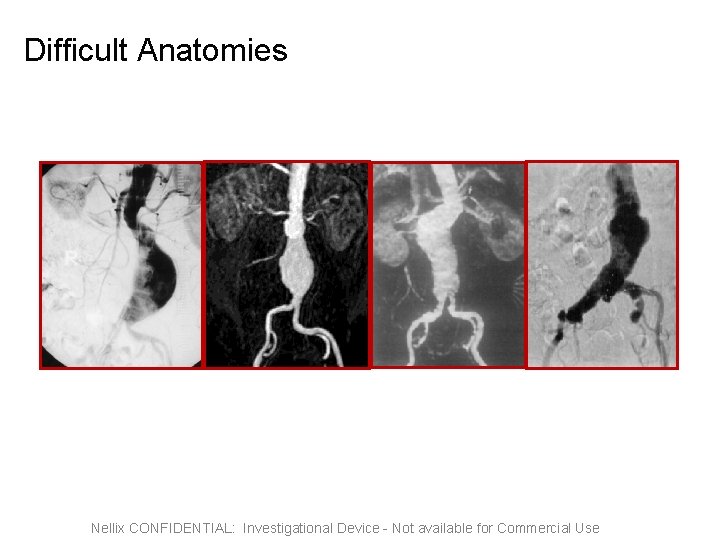
Difficult Anatomies Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

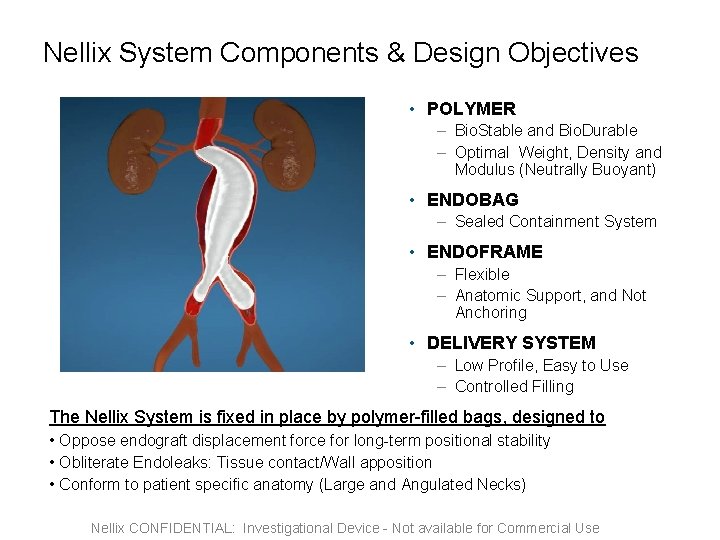
Nellix System Components & Design Objectives • POLYMER – Bio. Stable and Bio. Durable – Optimal Weight, Density and Modulus (Neutrally Buoyant) • ENDOBAG – Sealed Containment System • ENDOFRAME – Flexible – Anatomic Support, and Not Anchoring • DELIVERY SYSTEM – Low Profile, Easy to Use – Controlled Filling The Nellix System is fixed in place by polymer-filled bags, designed to • Oppose endograft displacement force for long-term positional stability • Obliterate Endoleaks: Tissue contact/Wall apposition • Conform to patient specific anatomy (Large and Angulated Necks) Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

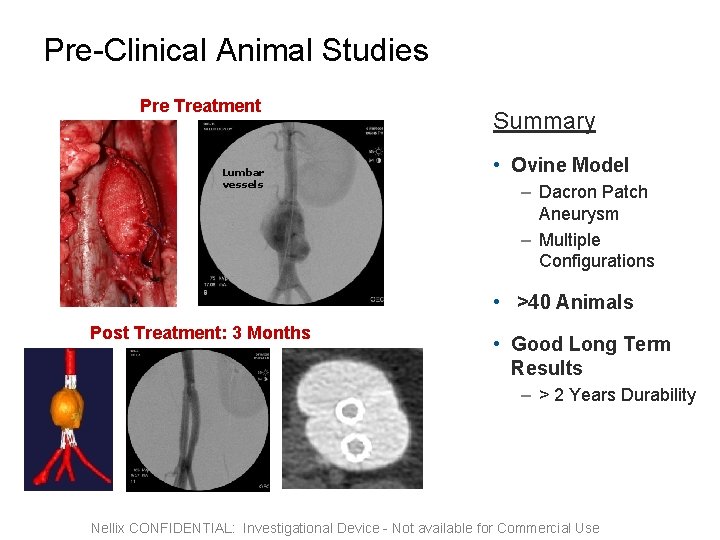
Pre-Clinical Animal Studies Pre Treatment Lumbar vessels Summary • Ovine Model – Dacron Patch Aneurysm – Multiple Configurations • >40 Animals Post Treatment: 3 Months • Good Long Term Results – > 2 Years Durability Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

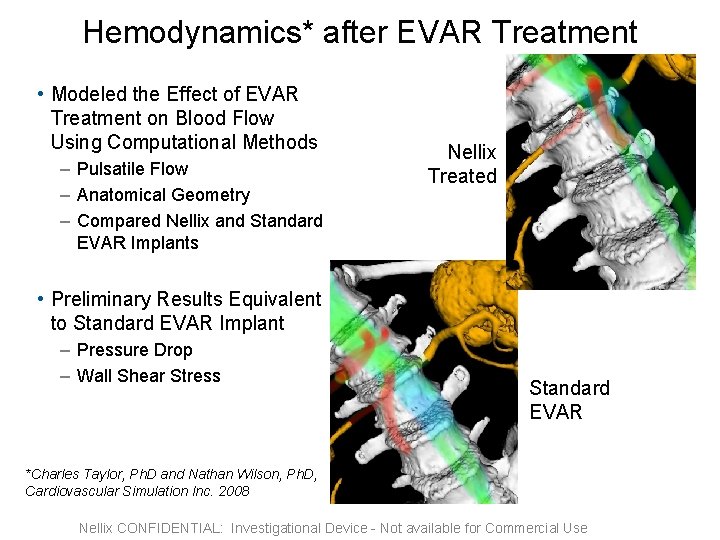
Hemodynamics* after EVAR Treatment • Modeled the Effect of EVAR Treatment on Blood Flow Using Computational Methods Nellix Treated – Pulsatile Flow – Anatomical Geometry – Compared Nellix and Standard EVAR Implants • Preliminary Results Equivalent to Standard EVAR Implant – Pressure Drop – Wall Shear Stress Standard EVAR *Charles Taylor, Ph. D and Nathan Wilson, Ph. D, Cardiovascular Simulation Inc. 2008 Confidential Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

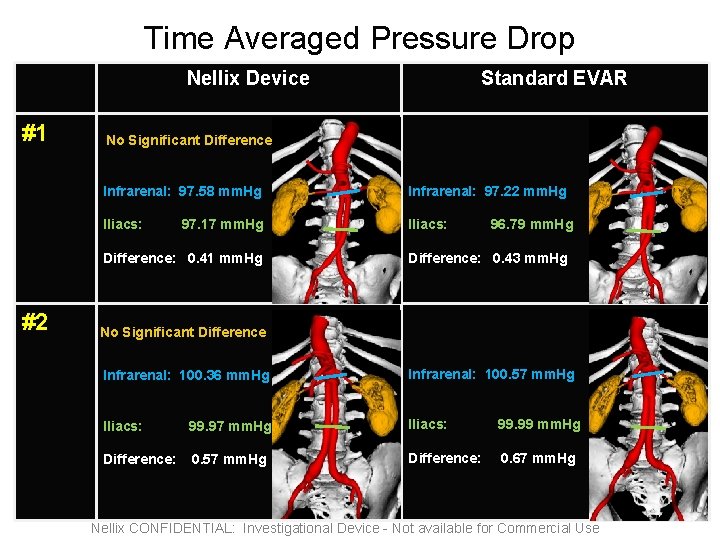
Time Averaged Pressure Drop Nellix Device #1 No Significant Difference Infrarenal: 97. 58 mm. Hg Infrarenal: 97. 22 mm. Hg Iliacs: 97. 17 mm. Hg Difference: 0. 41 mm. Hg #2 Standard EVAR 96. 79 mm. Hg Difference: 0. 43 mm. Hg No Significant Difference Infrarenal: 100. 36 mm. Hg Infrarenal: 100. 57 mm. Hg Iliacs: 99. 99 mm. Hg Difference: 0. 57 mm. Hg Difference: 0. 67 mm. Hg Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

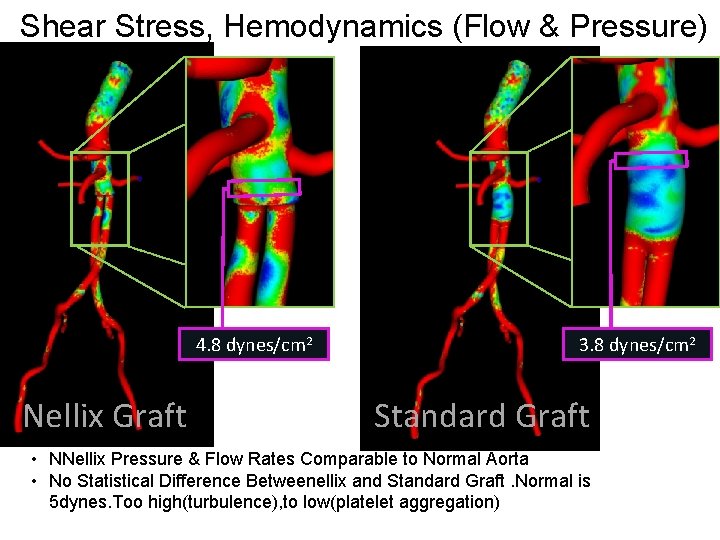
Shear Stress, Hemodynamics (Flow & Pressure) 4. 8 dynes/cm 2 Nellix Graft 3. 8 dynes/cm 2 Standard Graft • NNellix Pressure & Flow Rates Comparable to Normal Aorta • No Statistical Difference Betweenellix and Standard Graft. Normal is 5 dynes. Too high(turbulence), to low(platelet aggregation)

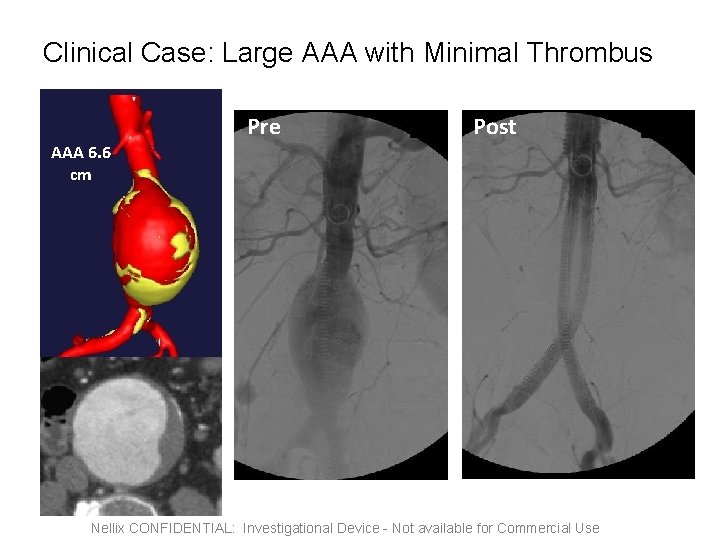
Clinical Case: Large AAA with Minimal Thrombus Pre Post AAA 6. 6 cm Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

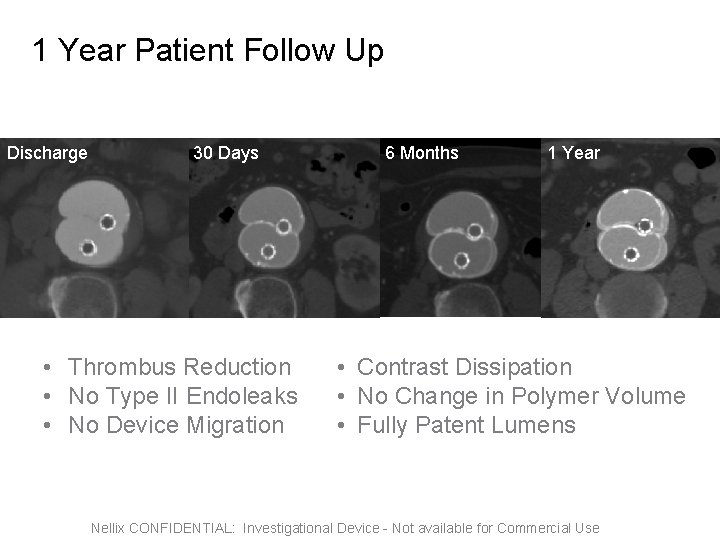
1 Year Patient Follow Up Discharge 30 Days • Thrombus Reduction • No Type II Endoleaks • No Device Migration 6 Months 1 Year 6 Month • Contrast Dissipation • No Change in Polymer Volume • Fully Patent Lumens Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

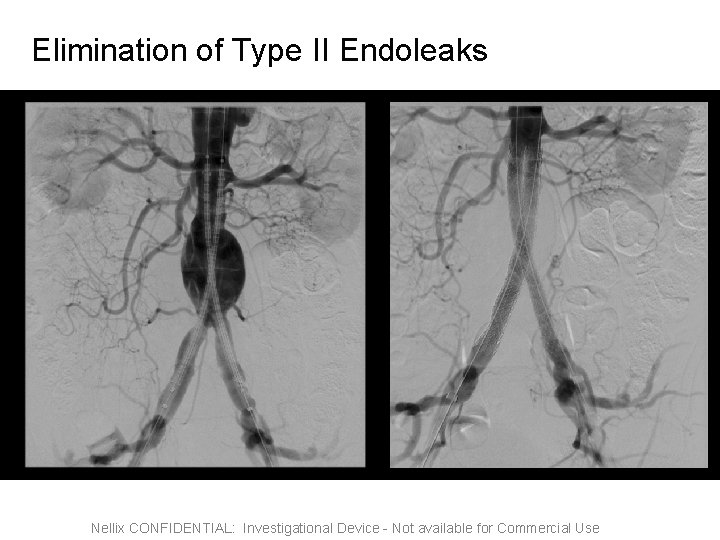
Elimination of Type II Endoleaks Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

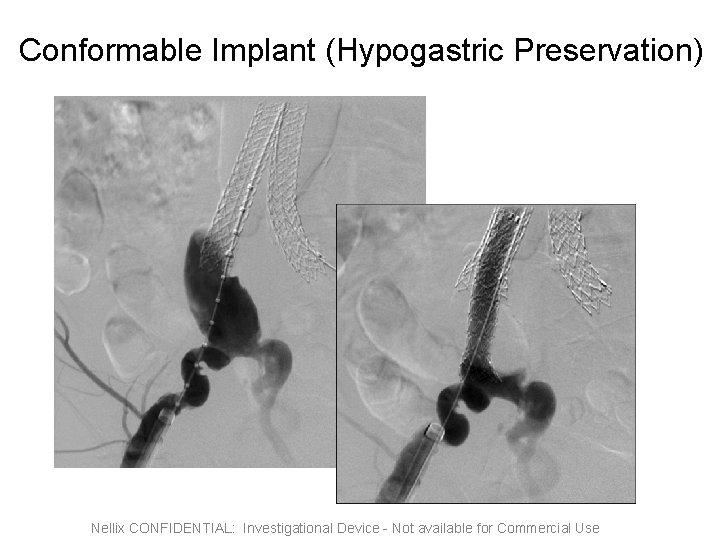
Conformable Implant (Hypogastric Preservation) Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use

Conclusions • Sac anchoring prosthesis is an innovative platform technology for endovascular treatment of aneurysms. • Preclinical bench and animal results have established the feasibility of this novel approach. • Initial clinical experience has been successful with the potential to treat challenging anatomies Nellix CONFIDENTIAL: Investigational Device - Not available for Commercial Use