RYDBERG ELECTRONS STEALTHY SPIES OF MOLECULAR STRUCTURE International

RYDBERG ELECTRONS STEALTHY SPIES OF MOLECULAR STRUCTURE International Symposium on Molecular Spectroscopy 17 June 2008 Michael P. Minitti

• • Rydberg States ion or molecule State of an where an excited electron has a high principal quantum number Hydrogenic in nature, with a binding energy given as: Rydberg I

Experimental Setup Ti: Sapphire BBO upconversion Regenerative Amplifier YLF Pump 4ω • 5 k. Hz rep. rate • 209 nm pump / 418 nm probe • 2ω e- MCPs ~230 fs 4ω pulse width CPU Timing Electronics Molecular beam Ion MCPs
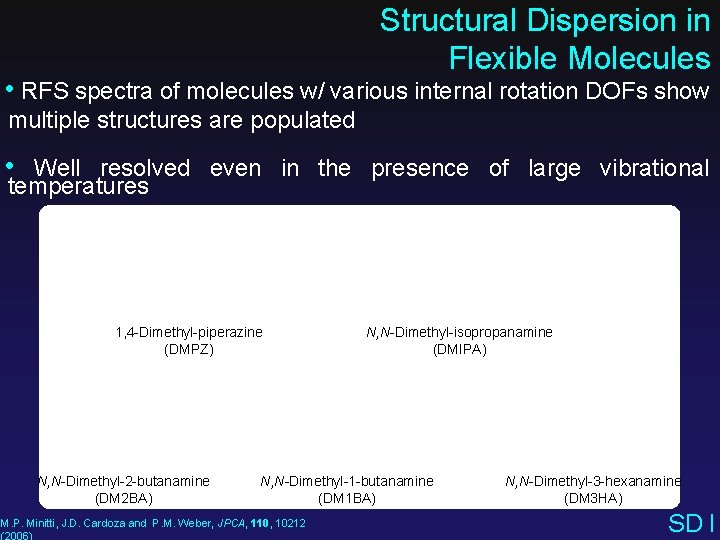
Structural Dispersion in Flexible Molecules • RFS spectra of molecules w/ various internal rotation DOFs show multiple structures are populated • Well resolved even in the presence of large vibrational temperatures 1, 4 -Dimethyl-piperazine (DMPZ) N, N-Dimethyl-2 -butanamine (DM 2 BA) N, N-Dimethyl-isopropanamine (DMIPA) N, N-Dimethyl-1 -butanamine (DM 1 BA) M. P. Minitti, J. D. Cardoza and P. M. Weber, JPCA, 110, 10212 (2006) N, N-Dimethyl-3 -hexanamine (DM 3 HA) SD I

Near time zero Intensity (arbitrary units) DM 3 HA DM 1 BA DM 2 BA 30 ps delay DMIPA DMPZ SD II

Vibrational Temperatures M. P. Minitti, J. D. Cardoza and P. M. Weber, JPCA, 110, 10212 SD III
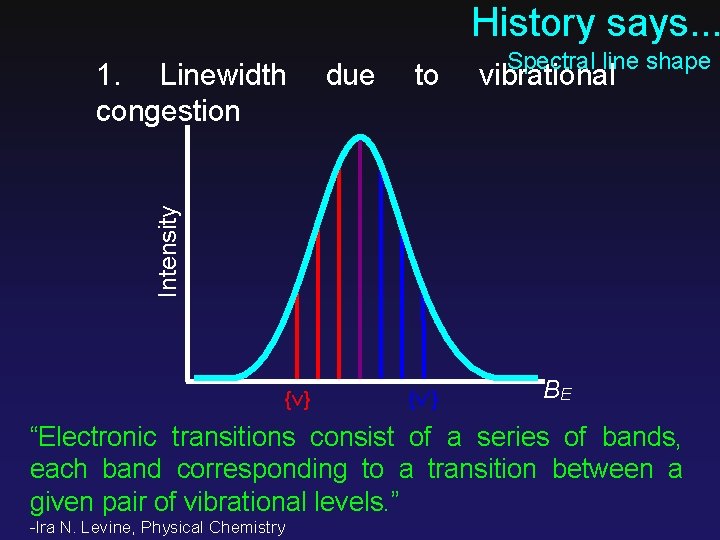
History says. . . due to vibrational Intensity 1. Linewidth congestion Spectral line shape {ν} {ν’} BE “Electronic transitions consist of a series of bands, each band corresponding to a transition between a given pair of vibrational levels. ” -Ira N. Levine, Physical Chemistry
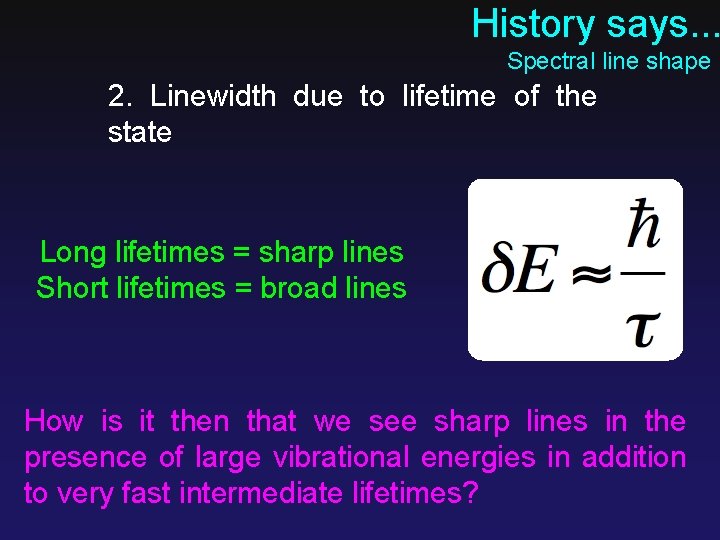
History says. . . Spectral line shape 2. Linewidth due to lifetime of the state Long lifetimes = sharp lines Short lifetimes = broad lines How is it then that we see sharp lines in the presence of large vibrational energies in addition to very fast intermediate lifetimes?
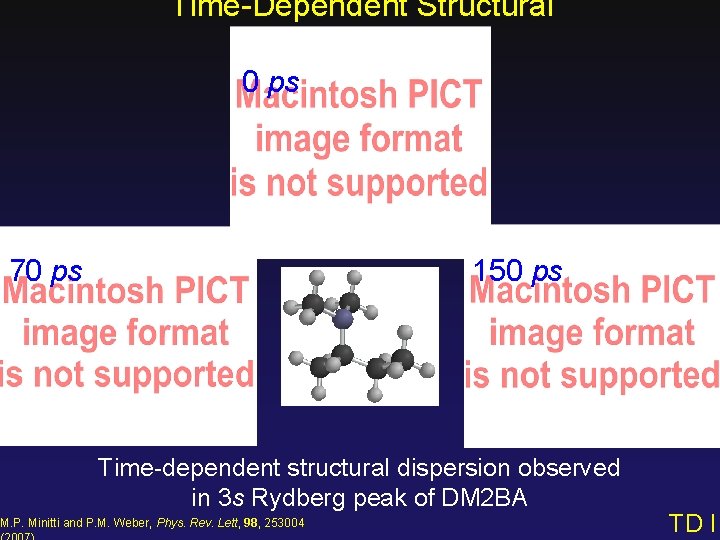
Time-Dependent Structural Dispersion 0 ps 70 ps 150 ps Time-dependent structural dispersion observed in 3 s Rydberg peak of DM 2 BA M. P. Minitti and P. M. Weber, Phys. Rev. Lett, 98, 253004 TD I

Experimentally determined fractional populations (area under the curves) Two dominant conformeric forms, A and B, in equilibrium via opposing first order reactions M. P. Minitti and P. M. Weber, Phys. Rev. Lett, 98, 253004 (2007) TD II

DFT Calculation Observed t = 0 fractional ground state population: 0. 67/0. 33 Calculated t = 0 fractional ground state population: 0. 65/0. 35 (using RT distributions) Observed t = ∞ fractional excited state population: 0. 78/0. 22 Calculated t = ∞ fractional excited state population: 0. 70/0. 30 (using previously estimated* vibrational temperature of 950 K) M. P. Minitti and P. M. Weber, Phys. Rev. Lett. , 98, 253004 * M. P. Minitti, J. D. Cardoza and P. M. Weber, J. Phys. Chem. A. , 110, 10212 (2006) TD III

Structural Dispersion Spectra are insensitive towards vibrational excitation and provide a purely electronic spectrum dependent on the coordinates of all electrons and nuclei and therefore the molecular structure What other spectral features can our Rydberg electron spies tell us about the molecular structure? NEXT MISSION: N, N, N’ - TMEDA
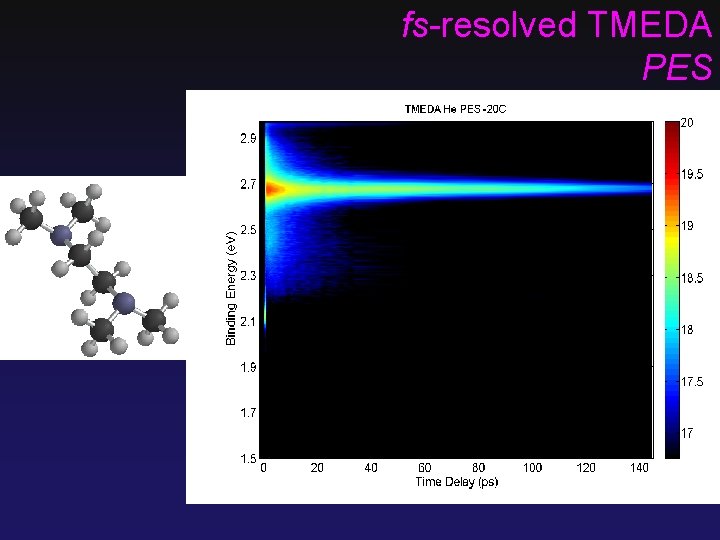
fs-resolved TMEDA PES
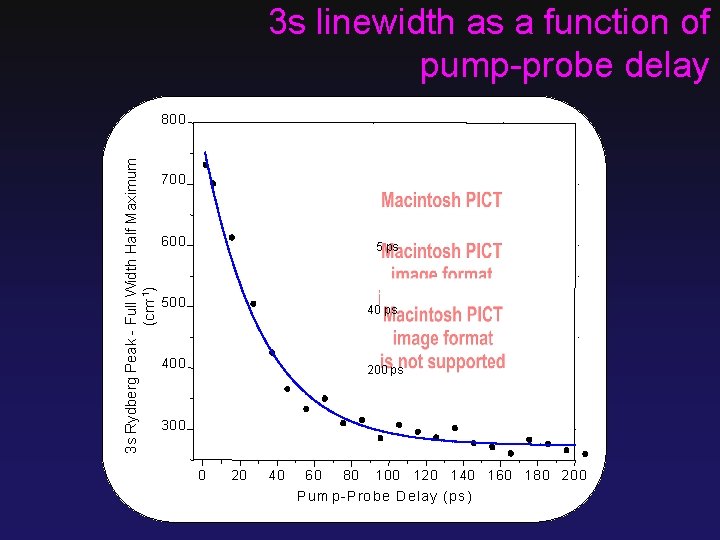
3 s linewidth as a function of pump-probe delay 3 s Rydberg Peak - Full Width Half Maximum (cm-1) 80 0 70 0 60 0 5 ps 50 0 40 ps 40 0 200 ps 30 0 0 20 40 60 80 1 0 0 12 0 1 40 1 6 0 1 8 0 2 0 0 P u m p -P ro b e D e lay (ps )
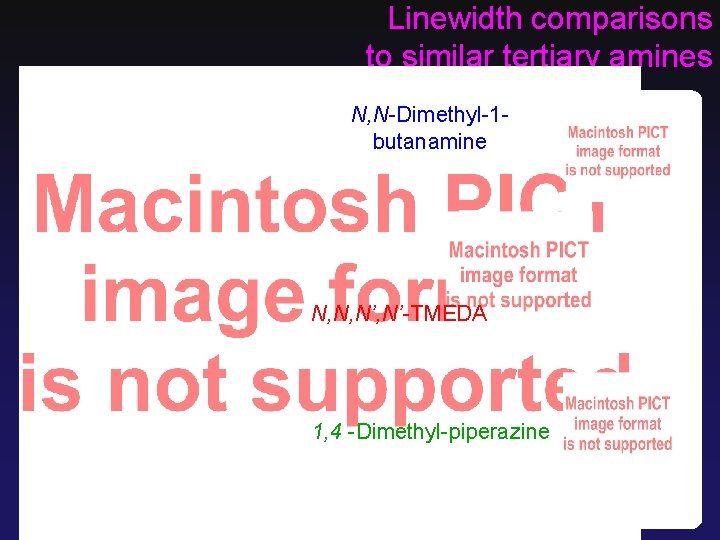
Linewidth comparisons to similar tertiary amines N, N-Dimethyl-1 butanamine N, N, N’-TMEDA 1, 4 -Dimethyl-piperazine

What’s the cause? TMEDA condenses in a minimum hν q configuration coordinate The molecule contains vibrational energies that are significant to the barriers in its energy landscape As vibrational energy dissipates, the molecule condenses in a minimum on its surface
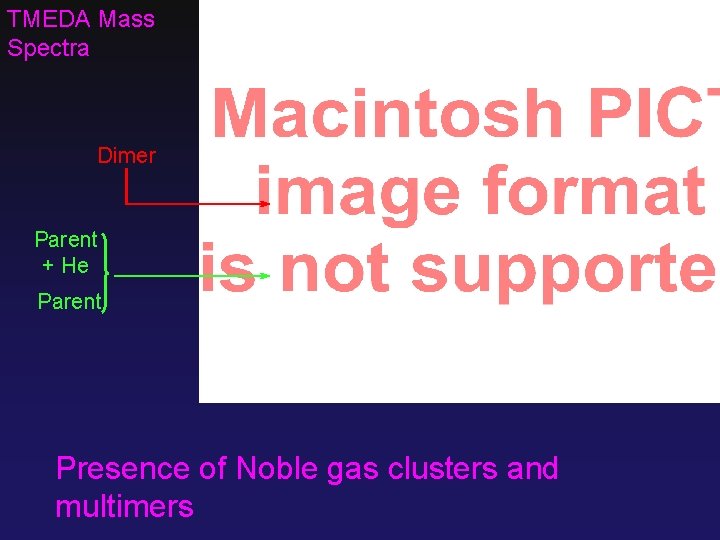
TMEDA Mass Spectra Dimer Parent + He Parent Presence of Noble gas clusters and multimers

Closing Remarks • Rydberg Fingerprint Spectroscopy has been proven to be sensitive to an array of molecular properties • Coupled with mass spectroscopy, RFS has multiplexing advantages • Chemically relevant systems can be investigated

Acknowledgement s • Prof. Peter Weber • Dr. Job Cardoza • Fedor Rudakov • Joe Bush • Sanghamitra Deb • Brad Taylor • Jie Bao • Brian Bayes $$$ DOE - Basic Sciences
- Slides: 19