RULES OF OXIDATION NUMBER ASSIGNMENT STEPS IN ASSIGNNING

RULES OF OXIDATION NUMBER ASSIGNMENT STEPS IN ASSIGNNING OXIDATION NUMBERS 1) IF THE PERIODIC TABLE GIVES ONLY ONE OXIDATION STATE, USE THAT STATE. EXAMPLE Zn+2. 2) APPLY THE RULES OF THIS POWERPOINT TO SOLVE FOR OXIDATION NUMBERS OF ELEMENTS WITH MULTIPLE OXIDATION STATES. 3) IF A MULTIPLE OXIDATION STATE ELEMENT HAS NO RULE, ASSIGN IT A VARIABLE AND SOLVE USIMNG THE ALGEBRA RULE(SLIDE 3).
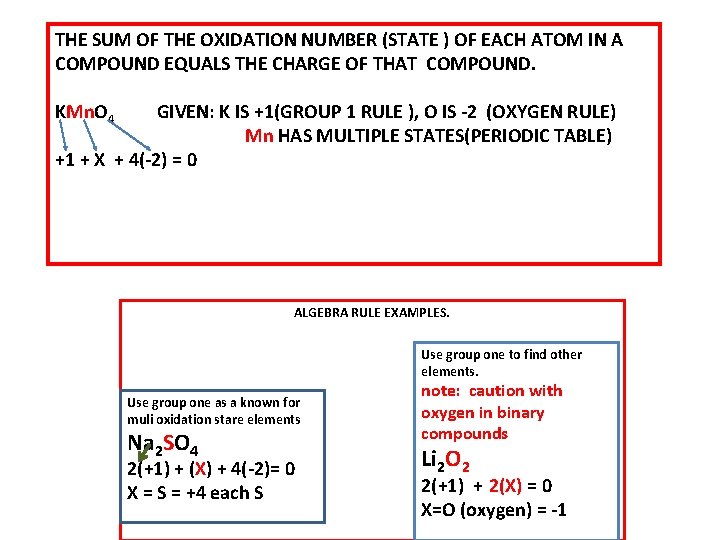
THE SUM OF THE OXIDATION NUMBER (STATE ) OF EACH ATOM IN A COMPOUND EQUALS THE CHARGE OF THAT COMPOUND. KMn. O 4 GIVEN: K IS +1(GROUP 1 RULE ), O IS -2 (OXYGEN RULE) Mn HAS MULTIPLE STATES(PERIODIC TABLE) +1 + X + 4(-2) = 0 ALGEBRA RULE EXAMPLES. Use group one to find other elements. Use group one as a known for muli oxidation stare elements Na 2 SO 4 2(+1) + (X) + 4(-2)= 0 X = S = +4 each S note: caution with oxygen in binary compounds Li 2 O 2 2(+1) + 2(X) = 0 X=O (oxygen) = -1
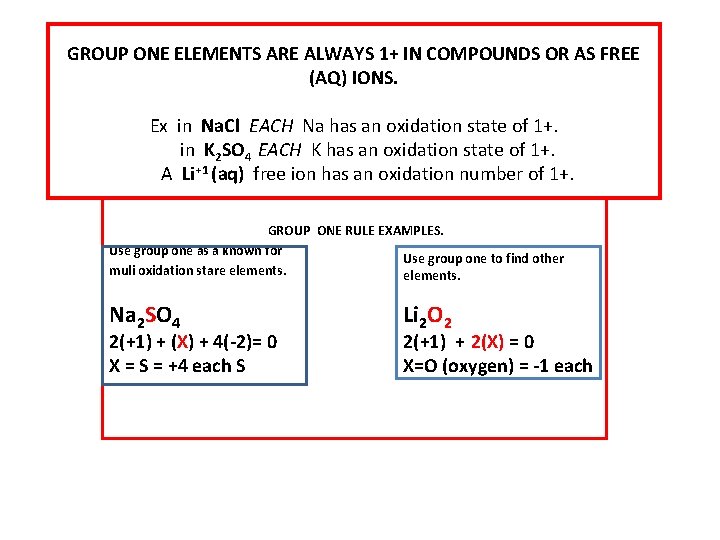
GROUP ONE ELEMENTS ARE ALWAYS 1+ IN COMPOUNDS OR AS FREE (AQ) IONS. Ex in Na. Cl EACH Na has an oxidation state of 1+. in K 2 SO 4 EACH K has an oxidation state of 1+. A Li+1 (aq) free ion has an oxidation number of 1+. GROUP ONE RULE EXAMPLES. Use group one as a known for Use group one to find other muli oxidation stare elements. Na 2 SO 4 2(+1) + (X) + 4(-2)= 0 X = S = +4 each S Li 2 O 2 2(+1) + 2(X) = 0 X=O (oxygen) = -1 each
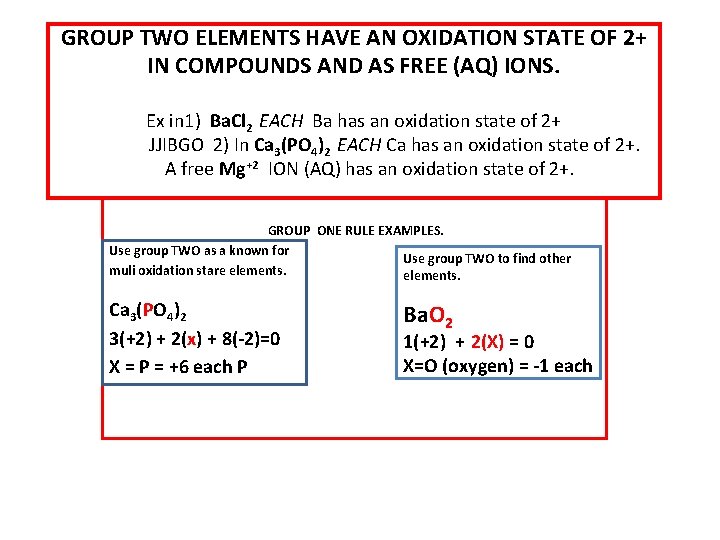
GROUP TWO ELEMENTS HAVE AN OXIDATION STATE OF 2+ IN COMPOUNDS AND AS FREE (AQ) IONS. Ex in 1) Ba. Cl 2 EACH Ba has an oxidation state of 2+ JJIBGO 2) In Ca 3(PO 4)2 EACH Ca has an oxidation state of 2+. A free Mg+2 ION (AQ) has an oxidation state of 2+. GROUP ONE RULE EXAMPLES. Use group TWO as a known for Use group TWO to find other muli oxidation stare elements. Ca 3(PO 4)2 3(+2) + 2(x) + 8(-2)=0 X = P = +6 each P Ba. O 2 1(+2) + 2(X) = 0 X=O (oxygen) = -1 each
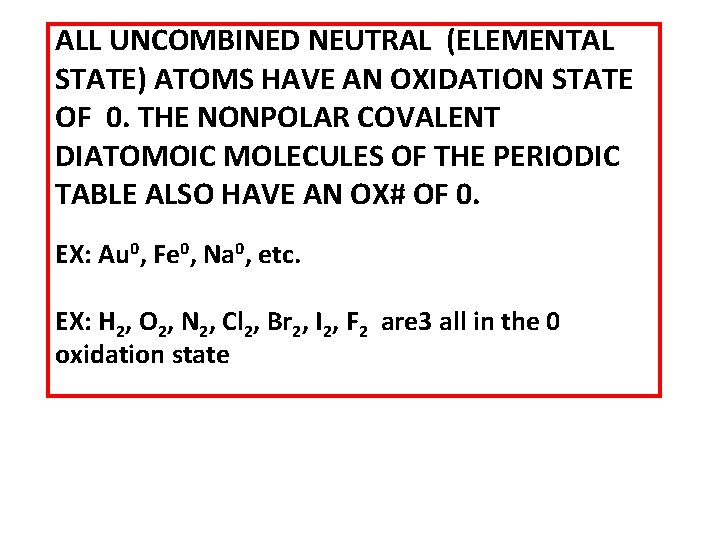
ALL UNCOMBINED NEUTRAL (ELEMENTAL STATE) ATOMS HAVE AN OXIDATION STATE OF 0. THE NONPOLAR COVALENT DIATOMOIC MOLECULES OF THE PERIODIC TABLE ALSO HAVE AN OX# OF 0. EX: Au 0, Fe 0, Na 0, etc. EX: H 2, O 2, N 2, Cl 2, Br 2, I 2, F 2 are 3 all in the 0 oxidation state
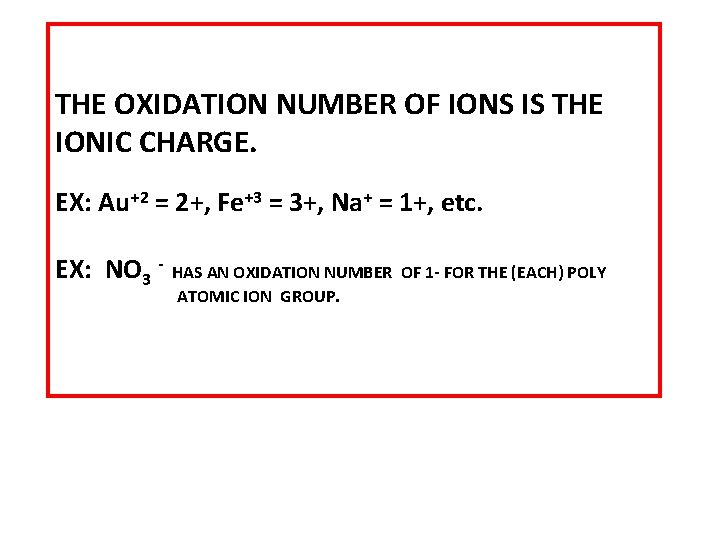
THE OXIDATION NUMBER OF IONS IS THE IONIC CHARGE. EX: Au+2 = 2+, Fe+3 = 3+, Na+ = 1+, etc. EX: NO 3 - HAS AN OXIDATION NUMBER OF 1 - FOR THE (EACH) POLY ATOMIC ION GROUP.
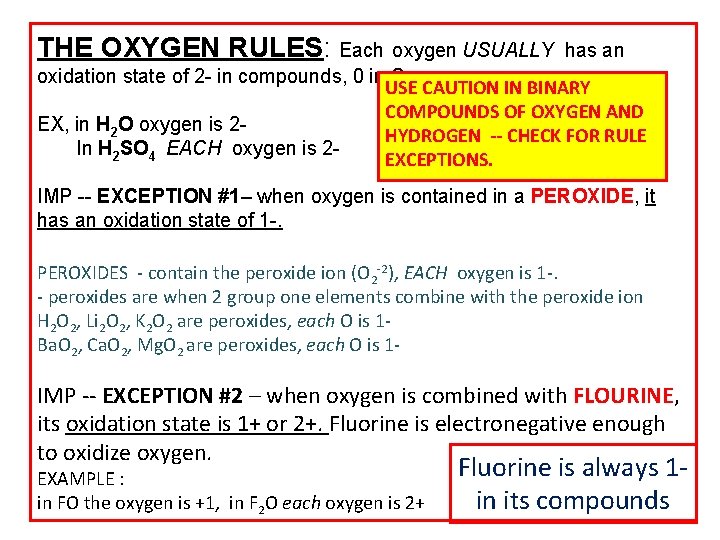
THE OXYGEN RULES: Each oxygen USUALLY has an oxidation state of 2 - in compounds, 0 in O 2. USE CAUTION IN BINARY COMPOUNDS OF OXYGEN AND EX, in H 2 O oxygen is 2 HYDROGEN -- CHECK FOR RULE In H 2 SO 4 EACH oxygen is 2 EXCEPTIONS. IMP -- EXCEPTION #1– when oxygen is contained in a PEROXIDE, it has an oxidation state of 1 -. PEROXIDES - contain the peroxide ion (O 2 -2), EACH oxygen is 1 -. - peroxides are when 2 group one elements combine with the peroxide ion H 2 O 2, Li 2 O 2, K 2 O 2 are peroxides, each O is 1 Ba. O 2, Ca. O 2, Mg. O 2 are peroxides, each O is 1 - IMP -- EXCEPTION #2 – when oxygen is combined with FLOURINE, its oxidation state is 1+ or 2+. Fluorine is electronegative enough to oxidize oxygen. EXAMPLE : in FO the oxygen is +1, in F 2 O each oxygen is 2+ Fluorine is always 1 in its compounds

THE HYDROGEN RULE: THE OXIDATION STATE OF HYDROGEN IS USUALLY 1+ EX in H 2 O each hydrogen is 1+ IMP-EXCEPTION – in GROUP 1 METAL HYDRIDES the hydrogen is 1 -, hydrogen is electronegative enough to oxidize group one metals. EX: Na. H, KH etc the hydrogen is 1 USE CAUTION IN BINARY COMPOUNDS OF OXYGEN AND HYDROGEN -- CHECK FOR RULE EXCEPTIONS.
- Slides: 8