Rules for Jeopardy 1 2 3 4 5























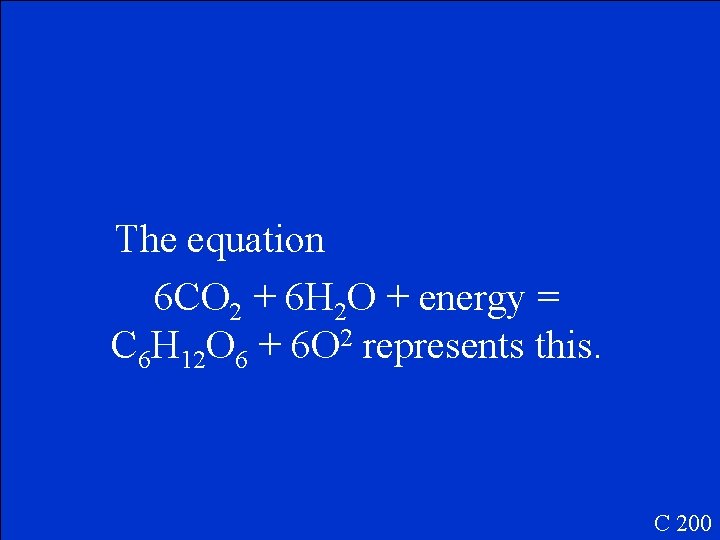













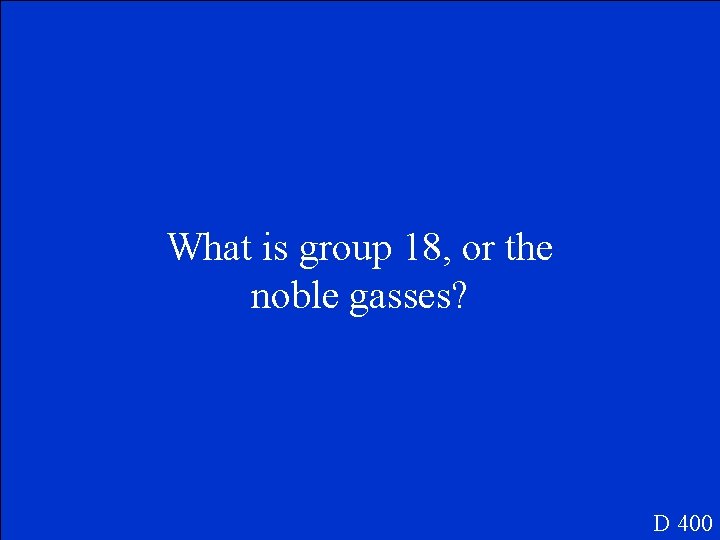






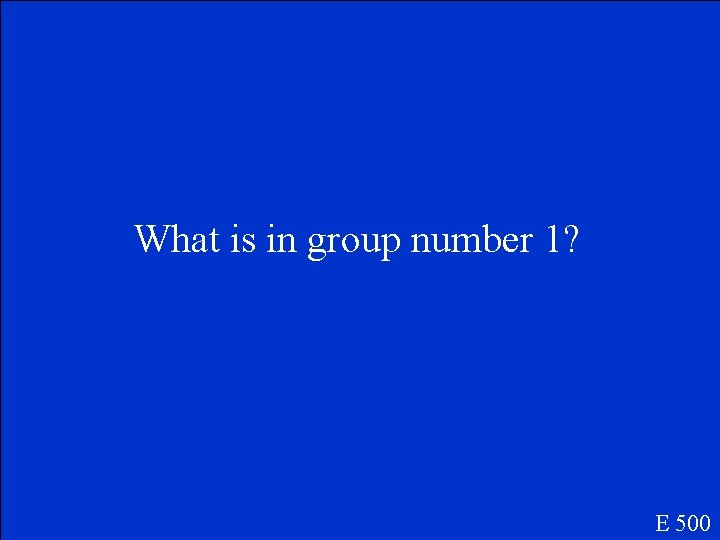












- Slides: 70

Rules for Jeopardy 1. 2. 3. 4. 5. 6. 7. 8. Each Group must designate a speaker, a time keeper, and a score keeper. Speaker: you are the only person allowed to give answers. If anyone else in your group answers (or is talking too loud) the question it will be treated as if your team gave an incorrect answer. Time Keeper: you will use the stop watch to keep time on all questions. Each Team will receive 30 seconds from the time the question has been read to answer. Score Keepers: you will keep track of all team scores (including your own). Remaining group members: each of the remaining group members can choose one (1) and only one resource in which to find answers. (resource examples: book, notes, quizzes, directed readings. ) You will have one opportunity to answer the question. If you miss the question you will loose the points from your cumulative score and it will be passed on to the next group to steal the points. If a question comes to you to steal the speaker may say “PASS” and you are not obligated to answer it. If your team tries to answer the question but gets it wrong you will loose those points as well. Questions?


THIS IS

With Your Host. . .

Earth Science 1 Earth Science 2 Physical Science 1 Physical Science 2 Physical Science 3 Life Science 1 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

Earth’s Seasons are due to this. A 100

What is Earth’s 23. 5 Degree Tilt as it orbits the sun? A 100

Earth’s rotation takes about this. A 200

What is 24 hours? A 200

These are the two factors that keep Earth and the moon in their orbits. A 300

What are gravity and inertia? A 300

This happens to air pressure as you rise upwards in the atmosphere. A 400

What is it decreases? A 400

This is the layer of the atmosphere that weather occurs in, and we also live in. A 500

What is the Troposphere? A 500

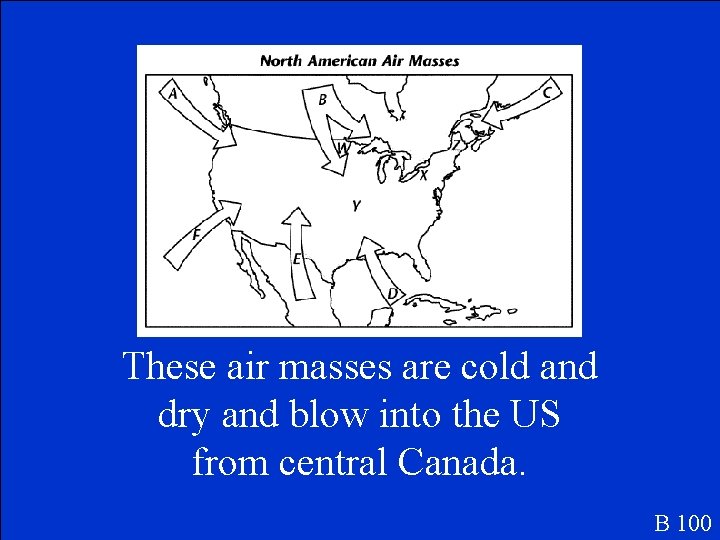
These air masses are cold and dry and blow into the US from central Canada. B 100

What are continental polar air masses? B 100

Weather associated with an anticyclone is generally this. B 200

What is dry and clear? B 200

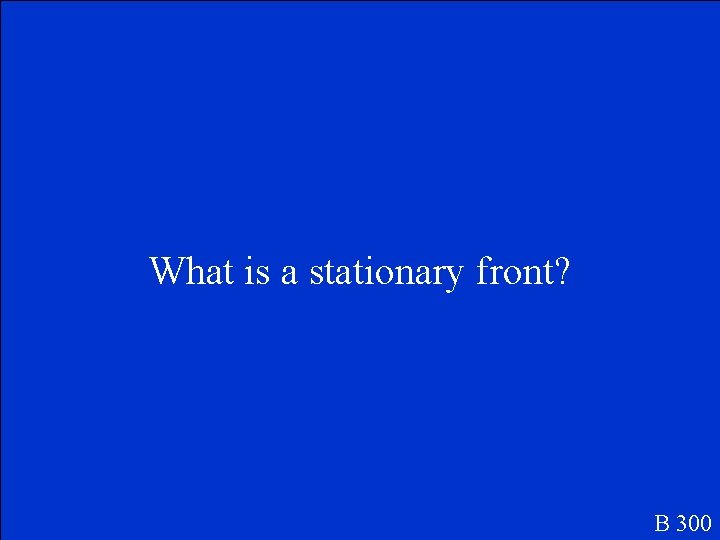
This is the term used to describe two air masses that meet and don’t move each other. B 300

What is a stationary front? B 300

The asteroid belt is located between these two planets. B 400

What are Jupiter and Mars? B 400

The sun-centered model of the universe is termed this. B 500

With is helio-centric? B 500

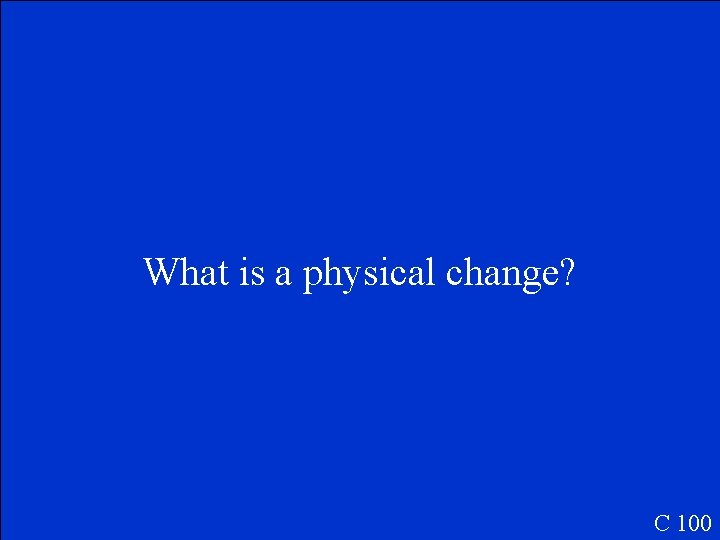
Dissolving salt in water is an example of this type of change. C 100

What is a physical change? C 100
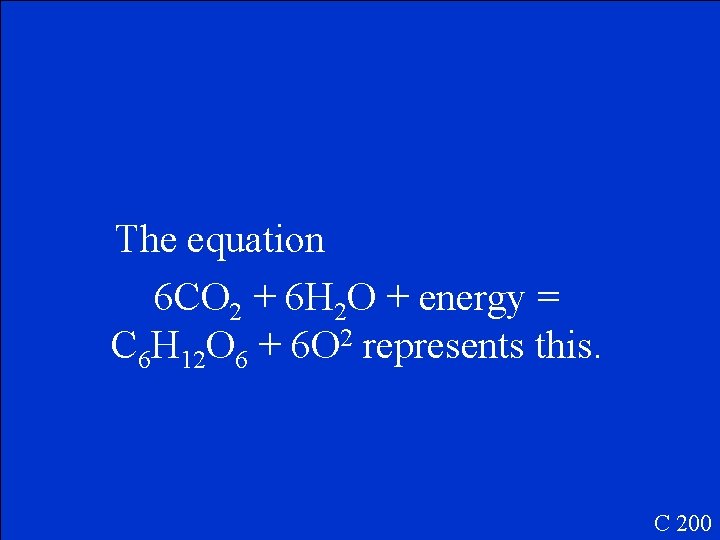
The equation 6 CO 2 + 6 H 2 O + energy = C 6 H 12 O 6 + 6 O 2 represents this. C 200

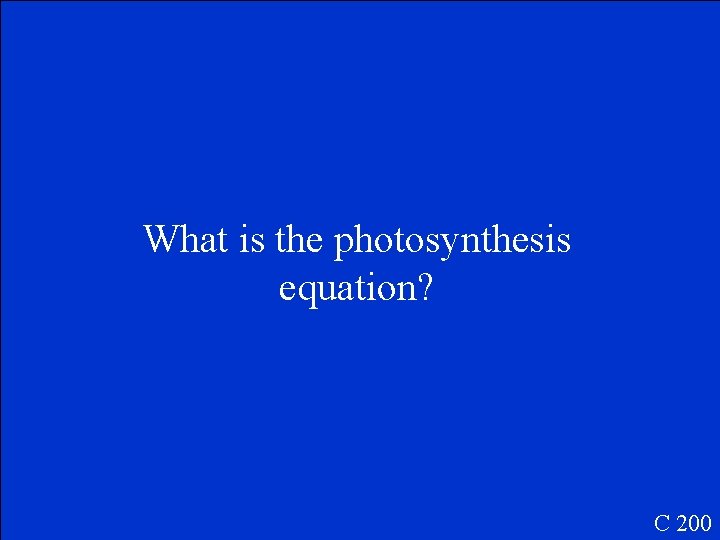
What is the photosynthesis equation? C 200

This is the ability to do work. C 300

What is energy? C 300

DAILY Place A Wager DOUBLE C 400

Burning wood is an example of this type of change. C 400

What is a chemical change? C 400

H 2 O, CO 2, and C 12 H 22 O 11 are all examples of these. C 500

What are chemical formulas? C 500

The volume of an irregular object can be measured by doing this. D 100

What is submerging it in water in a graduated cylinder and measuring the difference in the amount of water? D 100

This is the equation for measuring density. D 200

What is mass/volume? D 200

This increases as the speed of gas particles increases. D 300

What is temperature? D 300

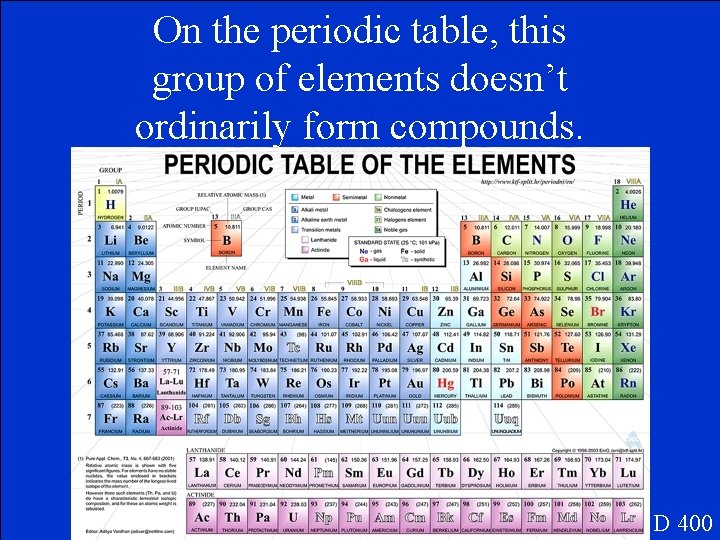
On the periodic table, this group of elements doesn’t ordinarily form compounds. D 400
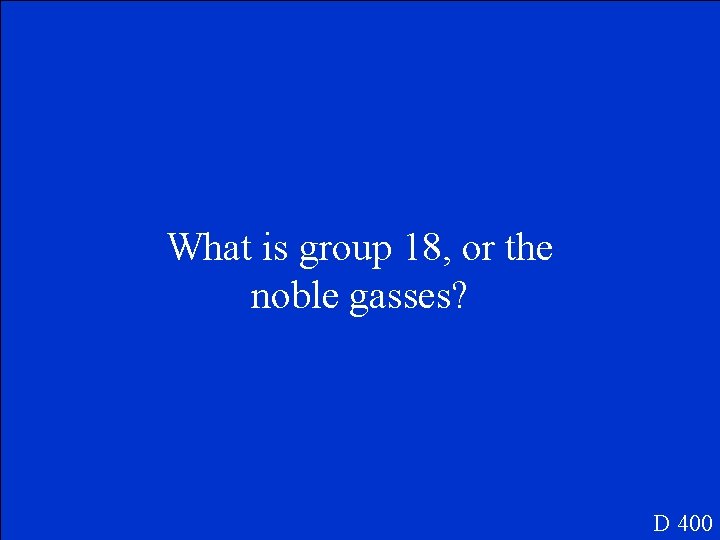
What is group 18, or the noble gasses? D 400

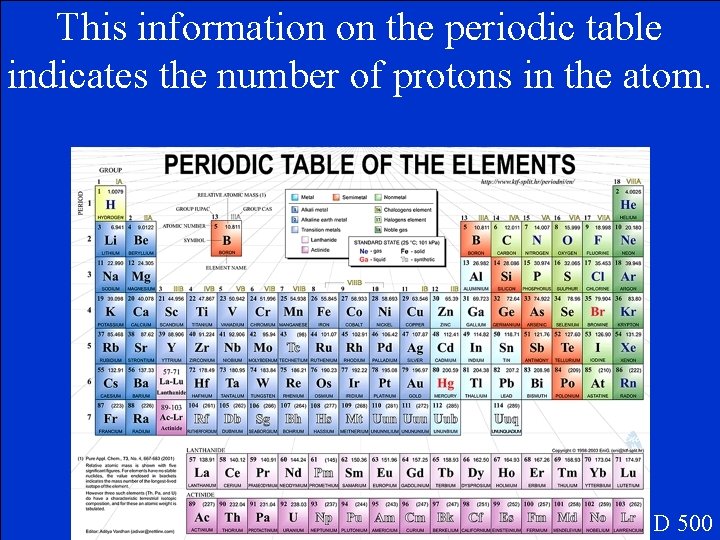
This information on the periodic table indicates the number of protons in the atom. D 500

What is the elements atomic number? D 500

This is the part of the atom that orbits the nucleus. E 100

What are the electrons? E 100

This is the sub atomic particle with a positive charge. E 200

What is the proton? E 200

When an atom loses an electron it becomes this. E 300

What is a positive ion? E 300

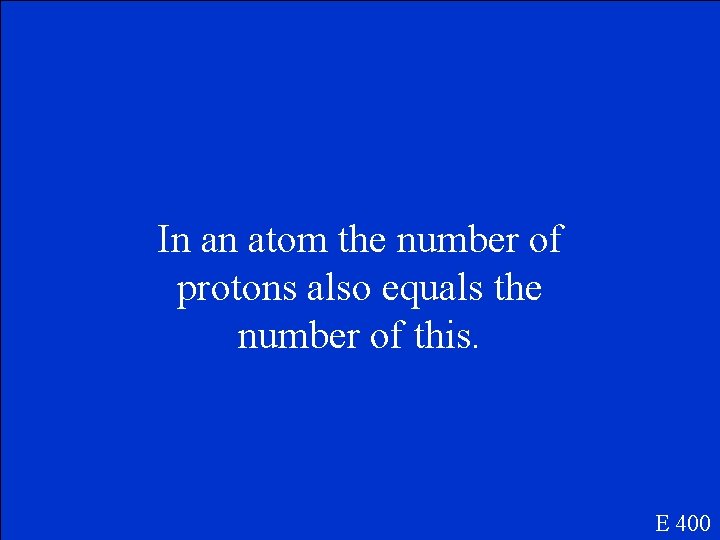
In an atom the number of protons also equals the number of this. E 400

What is the number of electrons? E 400

In the periodic table the most reactive atoms are located here? E 500
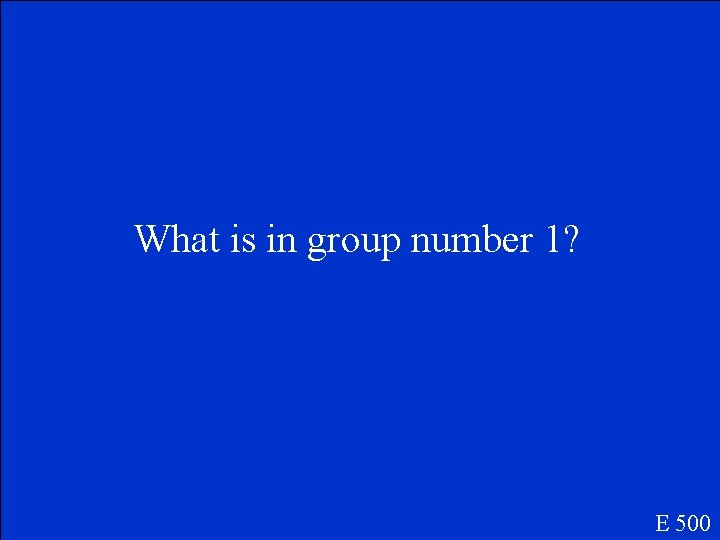
What is in group number 1? E 500

C 6 H 12 O 6 is the chemical formula for this substance. F 100

What is glucose? F 100

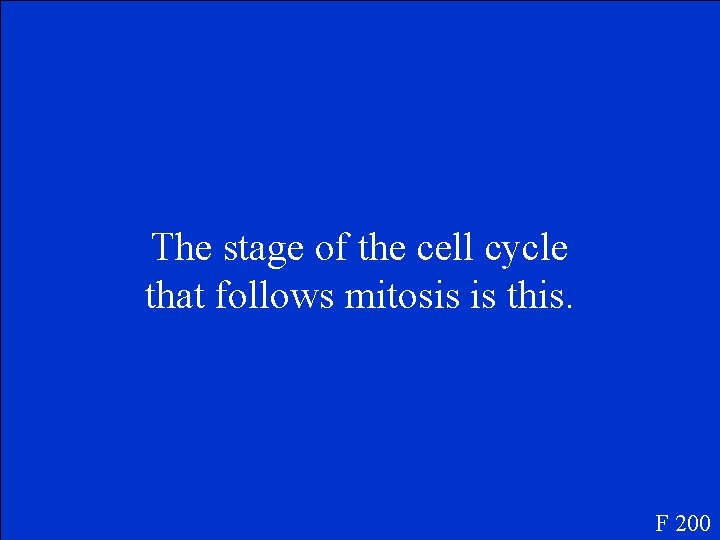
The stage of the cell cycle that follows mitosis is this. F 200

What is cytokinesis? F 200

Plants make their own food using energy that comes from this. F 300

What is the sun? F 300

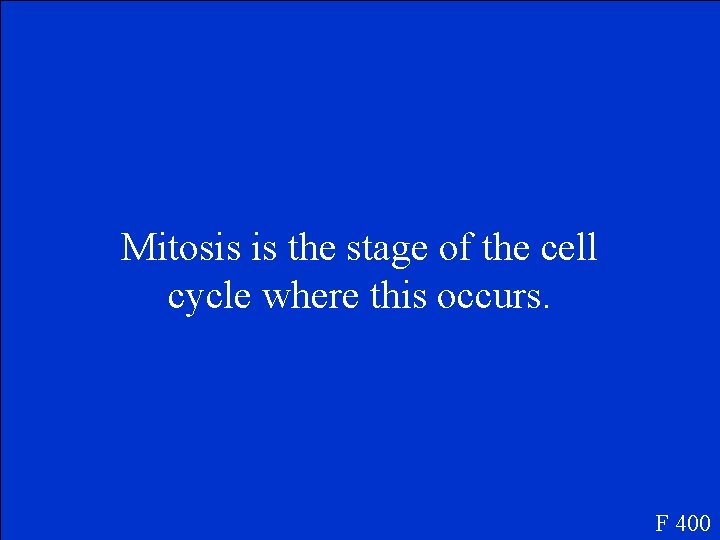
Mitosis is the stage of the cell cycle where this occurs. F 400

What is two new nuclei are created? F 400

This is copied during replication in interphase. F 500

What is DNA? F 500

The Final Jeopardy Category is: Lab Equipment Please record your wager. Click on screen to begin

Carol never wore these. Click on screen to continue

What are her safety goggles? Click on screen to continue

Thank You for Playing Jeopardy!