RTP ON DEMAND Multiple Myeloma Friday April 11

















- Slides: 50

RTP ON DEMAND: Multiple Myeloma Friday, April 11, 2014 Irene M Ghobrial, MD Dana-Farber/Harvard Cancer Center Ola Landgren, MD, Ph. D Center for Cancer Research National Cancer Institute

Case Presentation: 2 versus 3 Drug Pretransplant Induction Therapy


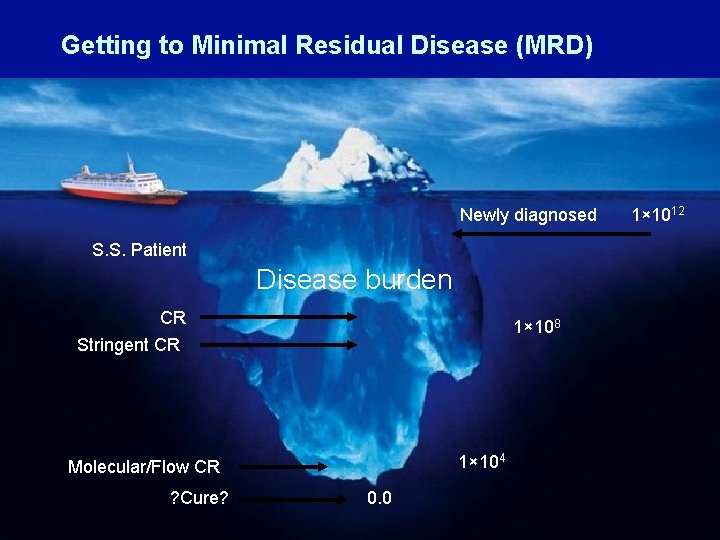
Getting to Minimal Residual Disease (MRD) Newly diagnosed S. S. Patient Disease burden CR 1× 108 Stringent CR 1× 104 Molecular/Flow CR ? Cure? 0. 0 1× 1012

Whole Exome Sequencing of Multiple Myeloma Reveals an Heterogeneous Clonal Architecture and Genomic Evolution Bolli N et al. Proc ASH 2013; Abstract 399. Serum Free Light Chain Escape in Progression and Treatment Resistance in Multiple Myeloma: A Marker for the Impact of Intra-Clonal Heterogeneity Brioli A et al. Proc ASH 2013; Abstract 752.

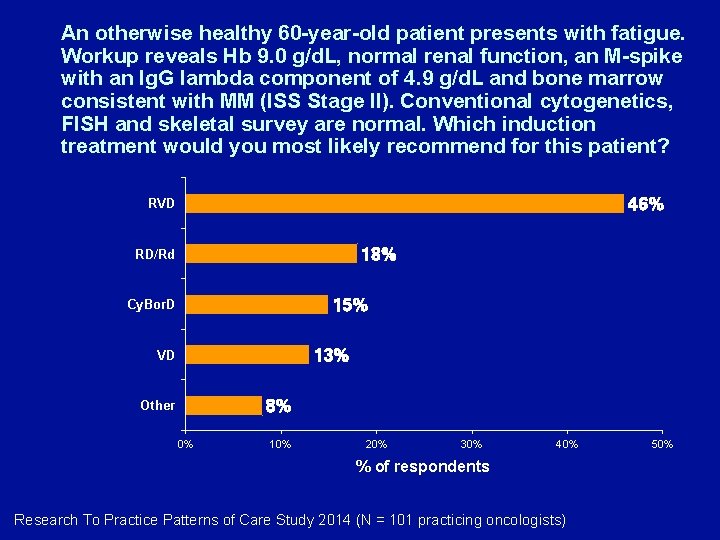
An otherwise healthy 60 -year-old patient presents with fatigue. Workup reveals Hb 9. 0 g/d. L, normal renal function, an M-spike with an Ig. G lambda component of 4. 9 g/d. L and bone marrow consistent with MM (ISS Stage II). Conventional cytogenetics, FISH and skeletal survey are normal. Which induction treatment would you most likely recommend for this patient? 46% RVD 18% RD/Rd 15% Cy. Bor. D 13% VD 8% Other 0% 10% 20% 30% 40% % of respondents Research To Practice Patterns of Care Study 2014 (N = 101 practicing oncologists) 50%

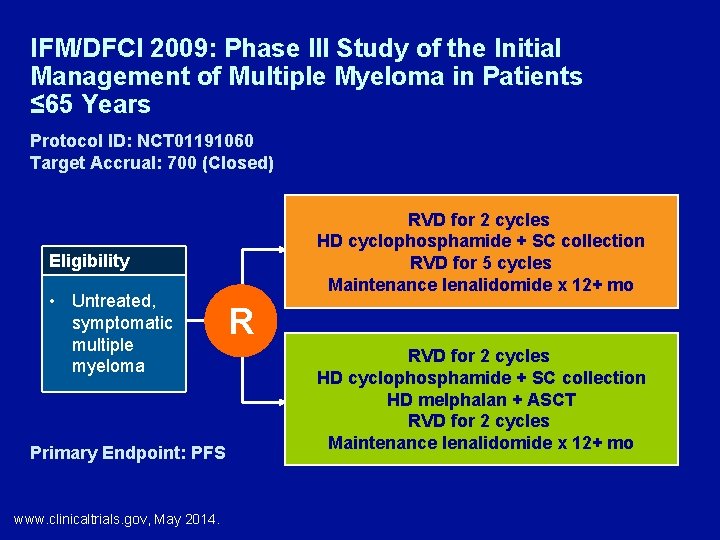
IFM/DFCI 2009: Phase III Study of the Initial Management of Multiple Myeloma in Patients ≤ 65 Years Protocol ID: NCT 01191060 Target Accrual: 700 (Closed) RVD for 2 cycles HD cyclophosphamide + SC collection RVD for 5 cycles Maintenance lenalidomide x 12+ mo Eligibility • Untreated, symptomatic multiple myeloma Primary Endpoint: PFS www. clinicaltrials. gov, May 2014. R RVD for 2 cycles HD cyclophosphamide + SC collection HD melphalan + ASCT RVD for 2 cycles Maintenance lenalidomide x 12+ mo

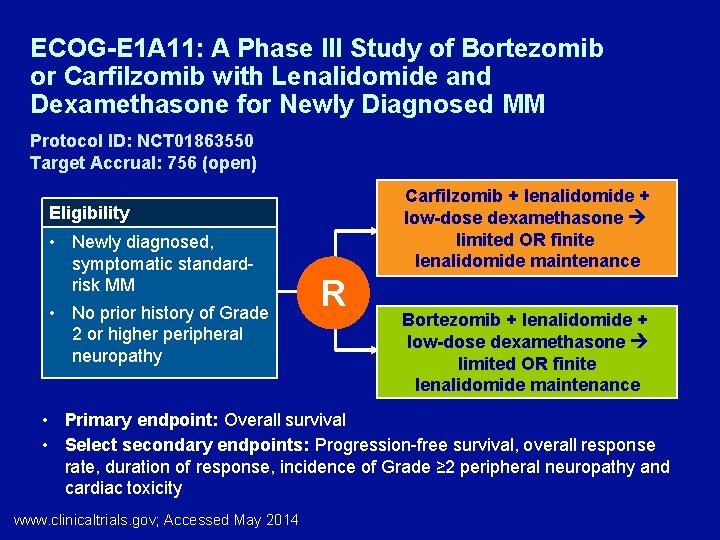
ECOG-E 1 A 11: A Phase III Study of Bortezomib or Carfilzomib with Lenalidomide and Dexamethasone for Newly Diagnosed MM Protocol ID: NCT 01863550 Target Accrual: 756 (open) Carfilzomib + lenalidomide + low-dose dexamethasone limited OR finite lenalidomide maintenance Eligibility • Newly diagnosed, symptomatic standardrisk MM • No prior history of Grade 2 or higher peripheral neuropathy R Bortezomib + lenalidomide + low-dose dexamethasone limited OR finite lenalidomide maintenance • Primary endpoint: Overall survival • Select secondary endpoints: Progression-free survival, overall response rate, duration of response, incidence of Grade ≥ 2 peripheral neuropathy and cardiac toxicity www. clinicaltrials. gov; Accessed May 2014

Phase II Clinical and Correlative Study of Carfilzomib, Lenalidomide, and Dexamethasone Followed by Lenalidomide Extended Dosing (CRd-R) Induces High Rates of MRD Negativity in Newly Diagnosed Multiple Myeloma (MM) Patients Korde N et al. Proc ASH 2013; Abstract 538.

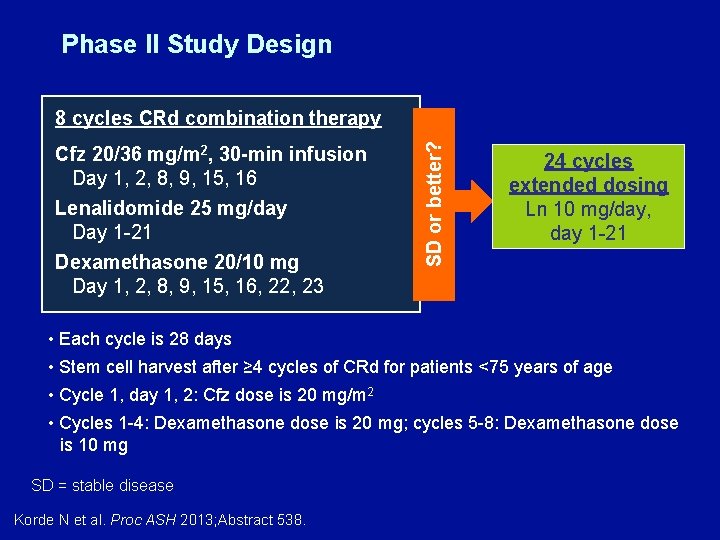
Phase II Study Design Cfz 20/36 mg/m 2, 30 -min infusion Day 1, 2, 8, 9, 15, 16 Lenalidomide 25 mg/day Day 1 -21 Dexamethasone 20/10 mg Day 1, 2, 8, 9, 15, 16, 22, 23 SD or better? 8 cycles CRd combination therapy 24 cycles extended dosing Ln 10 mg/day, day 1 -21 • Each cycle is 28 days • Stem cell harvest after ≥ 4 cycles of CRd for patients <75 years of age • Cycle 1, day 1, 2: Cfz dose is 20 mg/m 2 • Cycles 1 -4: Dexamethasone dose is 20 mg; cycles 5 -8: Dexamethasone dose is 10 mg SD = stable disease Korde N et al. Proc ASH 2013; Abstract 538.

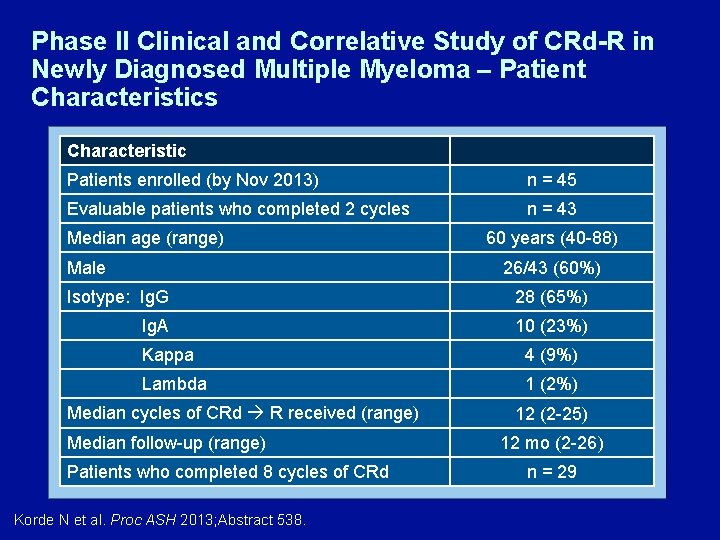
Phase II Clinical and Correlative Study of CRd-R in Newly Diagnosed Multiple Myeloma – Patient Characteristics Characteristic Patients enrolled (by Nov 2013) n = 45 Evaluable patients who completed 2 cycles n = 43 Median age (range) Male 60 years (40 -88) 26/43 (60%) Isotype: Ig. G 28 (65%) Ig. A 10 (23%) Kappa 4 (9%) Lambda 1 (2%) Median cycles of CRd R received (range) Median follow-up (range) Patients who completed 8 cycles of CRd Korde N et al. Proc ASH 2013; Abstract 538. 12 (2 -25) 12 mo (2 -26) n = 29

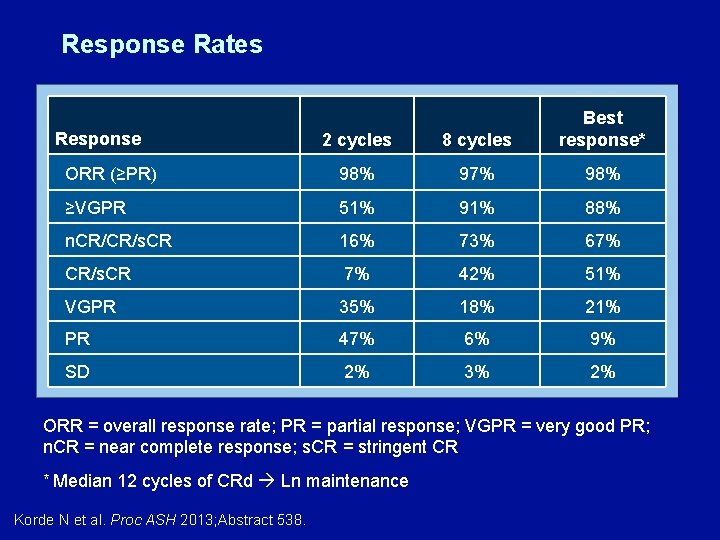
Response Rates 2 cycles 8 cycles Best response* ORR (≥PR) 98% 97% 98% ≥VGPR 51% 91% 88% n. CR/CR/s. CR 16% 73% 67% CR/s. CR 7% 42% 51% VGPR 35% 18% 21% PR 47% 6% 9% SD 2% 3% 2% Response ORR = overall response rate; PR = partial response; VGPR = very good PR; n. CR = near complete response; s. CR = stringent CR * Median 12 cycles of CRd Ln maintenance Korde N et al. Proc ASH 2013; Abstract 538.

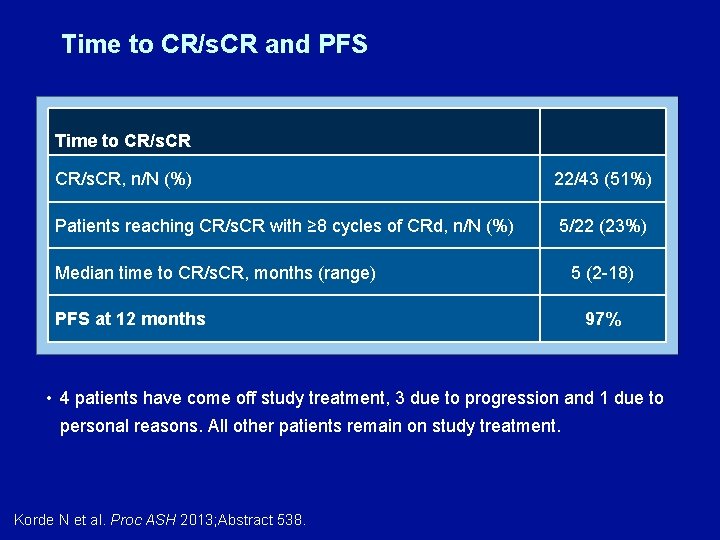
Time to CR/s. CR and PFS Time to CR/s. CR, n/N (%) 22/43 (51%) Patients reaching CR/s. CR with ≥ 8 cycles of CRd, n/N (%) 5/22 (23%) Median time to CR/s. CR, months (range) PFS at 12 months 5 (2 -18) 97% • 4 patients have come off study treatment, 3 due to progression and 1 due to personal reasons. All other patients remain on study treatment. Korde N et al. Proc ASH 2013; Abstract 538.

Immunophenotypic Evaluation of the Plasma Cell Compartment in Multiple Myeloma: A Tool for Comparing the Efficacy of Different Treatment Strategies and Predicting Outcome San Miguel JF et al. Blood 2002; 99(5): 1853 -56.

Martinez-Lopez J et al. Blood 2014; 123(20): 3073 -9.

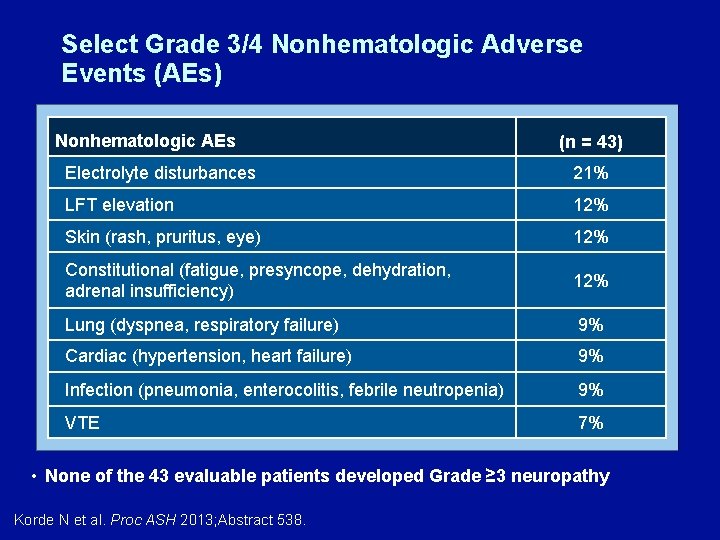
Select Grade 3/4 Nonhematologic Adverse Events (AEs) Nonhematologic AEs (n = 43) Electrolyte disturbances 21% LFT elevation 12% Skin (rash, pruritus, eye) 12% Constitutional (fatigue, presyncope, dehydration, adrenal insufficiency) 12% Lung (dyspnea, respiratory failure) 9% Cardiac (hypertension, heart failure) 9% Infection (pneumonia, enterocolitis, febrile neutropenia) 9% VTE 7% • None of the 43 evaluable patients developed Grade ≥ 3 neuropathy Korde N et al. Proc ASH 2013; Abstract 538.

Predictors of Treatment Outcome with the Combination of Carfilzomib, Lenalidomide, and Low-Dose Dexamethasone (CRd) in Newly Diagnosed Multiple Myeloma (NDMM) Jasielec J et al. Proc ASH 2013; Abstract 3220.

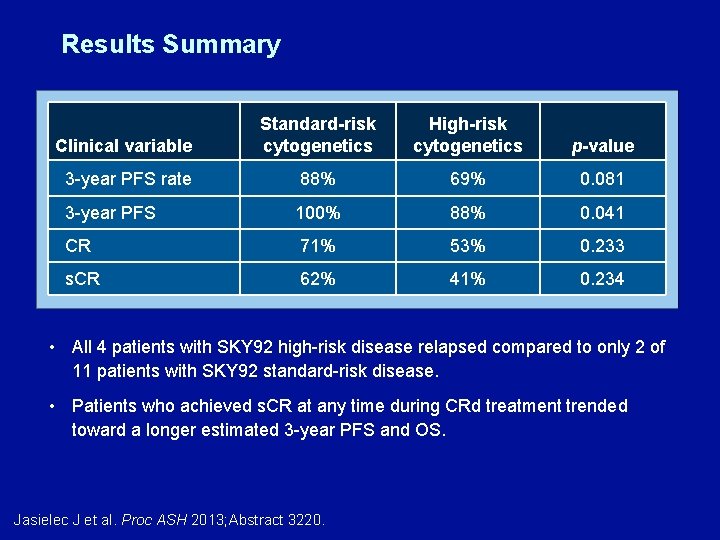
Results Summary Clinical variable Standard-risk cytogenetics High-risk cytogenetics p-value 3 -year PFS rate 88% 69% 0. 081 3 -year PFS 100% 88% 0. 041 CR 71% 53% 0. 233 s. CR 62% 41% 0. 234 • All 4 patients with SKY 92 high-risk disease relapsed compared to only 2 of 11 patients with SKY 92 standard-risk disease. • Patients who achieved s. CR at any time during CRd treatment trended toward a longer estimated 3 -year PFS and OS. Jasielec J et al. Proc ASH 2013; Abstract 3220.

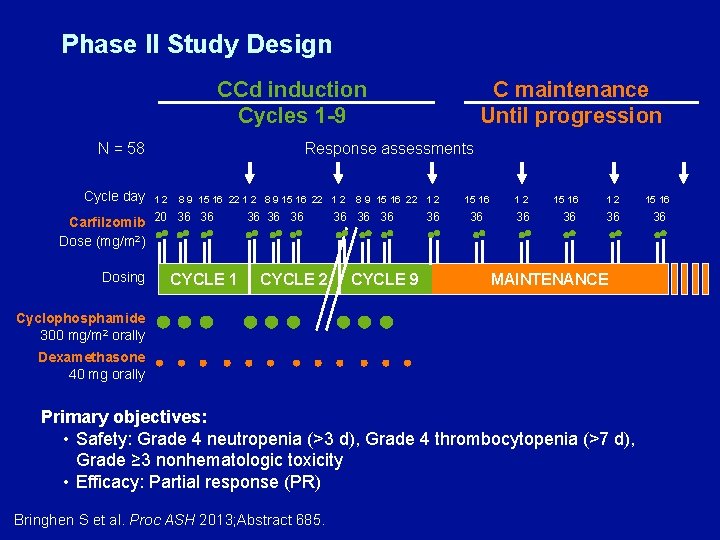
A Phase II Study with Carfilzomib, Cyclophosphamide, and Dexamethasone (CCd) for Newly Diagnosed Multiple Myeloma Bringhen S et al. Proc ASH 2013; Abstract 685.

Phase II Study Design CCd induction Cycles 1 -9 N = 58 Cycle day C maintenance Until progression Response assessments 1 2 8 9 15 16 22 1 2 15 16 Carfilzomib 20 36 36 36 36 36 Dose (mg/m 2) Dosing CYCLE 1 CYCLE 2 CYCLE 9 MAINTENANCE Cyclophosphamide 300 mg/m 2 orally Dexamethasone 40 mg orally Primary objectives: • Safety: Grade 4 neutropenia (>3 d), Grade 4 thrombocytopenia (>7 d), Grade ≥ 3 nonhematologic toxicity • Efficacy: Partial response (PR) Bringhen S et al. Proc ASH 2013; Abstract 685.

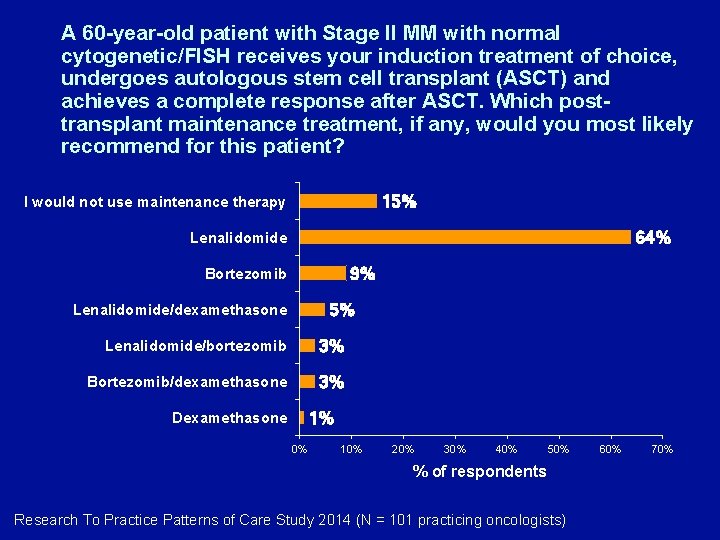
A 60 -year-old patient with Stage II MM with normal cytogenetic/FISH receives your induction treatment of choice, undergoes autologous stem cell transplant (ASCT) and achieves a complete response after ASCT. Which posttransplant maintenance treatment, if any, would you most likely recommend for this patient? 15% I would not use maintenance therapy 64% Lenalidomide 9% Bortezomib 5% Lenalidomide/dexamethasone Lenalidomide/bortezomib 3% Bortezomib/dexamethasone 3% 1% Dexamethasone 0% 10% 20% 30% 40% 50% % of respondents Research To Practice Patterns of Care Study 2014 (N = 101 practicing oncologists) 60% 70%

N Engl J Med 2012; 366: 1782 -91. N Engl J Med 2012; 366: 1770 -81.

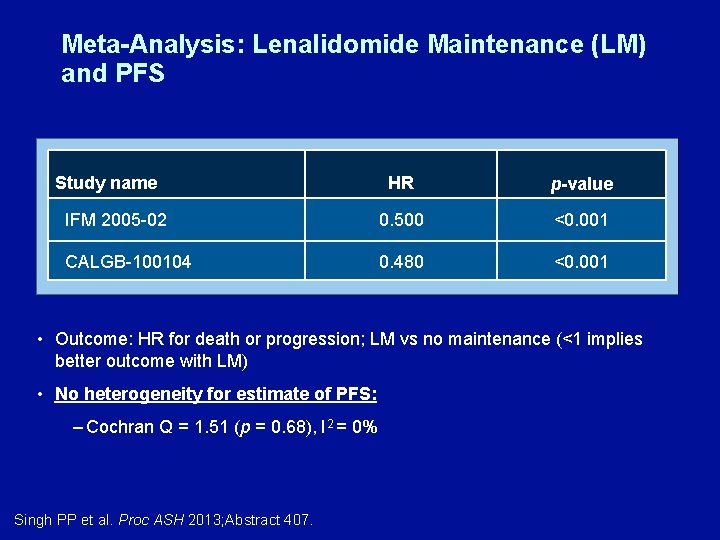
Meta-Analysis: Lenalidomide Maintenance (LM) and PFS Study name HR p-value IFM 2005 -02 0. 500 <0. 001 CALGB-100104 0. 480 <0. 001 • Outcome: HR for death or progression; LM vs no maintenance (<1 implies better outcome with LM) • No heterogeneity for estimate of PFS: – Cochran Q = 1. 51 (p = 0. 68), I 2 = 0% Singh PP et al. Proc ASH 2013; Abstract 407.

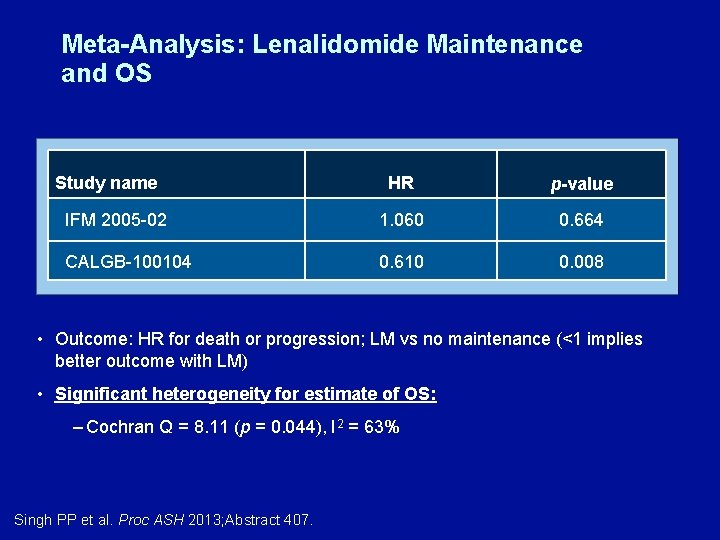
Meta-Analysis: Lenalidomide Maintenance and OS Study name HR p-value IFM 2005 -02 1. 060 0. 664 CALGB-100104 0. 610 0. 008 • Outcome: HR for death or progression; LM vs no maintenance (<1 implies better outcome with LM) • Significant heterogeneity for estimate of OS: – Cochran Q = 8. 11 (p = 0. 044), I 2 = 63% Singh PP et al. Proc ASH 2013; Abstract 407.

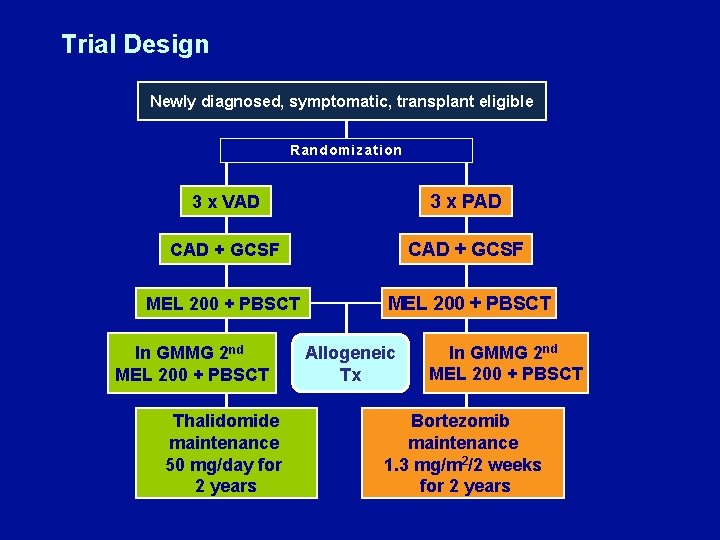
Bortezomib Induction and Maintenance Treatment Improves Survival in Patients with Newly Diagnosed Multiple Myeloma: Extended Follow-Up of the HOVON-65/GMMG-HD 4 Trial Sonneveld P et al. Proc ASH 2013; Abstract 404.

Trial Design Newly diagnosed, symptomatic, transplant eligible Randomization 3 x VAD 3 x PAD CAD + GCSF MEL 200 + PBSCT In GMMG 2 nd MEL 200 + PBSCT Thalidomide maintenance 50 mg/day for 2 years Allogeneic Tx In GMMG 2 nd MEL 200 + PBSCT Bortezomib maintenance 1. 3 mg/m 2/2 weeks for 2 years

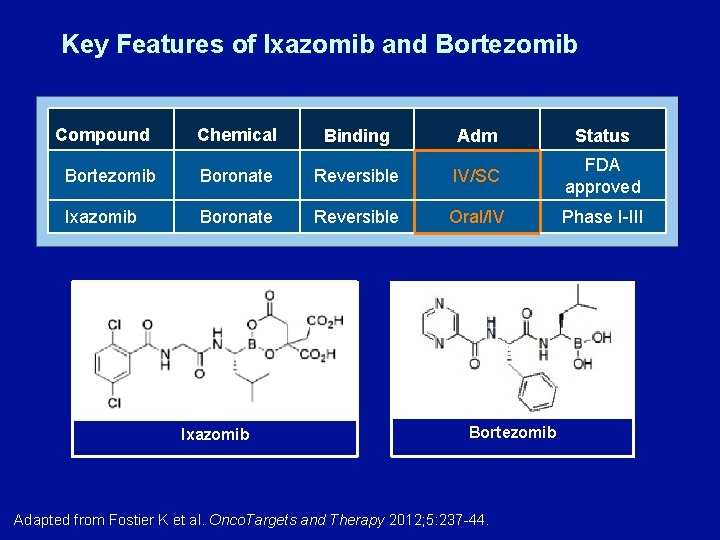
Key Features of Ixazomib and Bortezomib Compound Chemical Binding Adm Status Bortezomib Boronate Reversible IV/SC FDA approved Ixazomib Boronate Reversible Oral/IV Phase I-III Ixazomib Bortezomib Adapted from Fostier K et al. Onco. Targets and Therapy 2012; 5: 237 -44.

Twice-Weekly Oral MLN 9708 (Ixazomib Citrate), an Investigational Proteasome Inhibitor, in Combination with Lenalidomide (Len) and Dexamethasone (Dex) in Patients (Pts) with Newly Diagnosed Multiple Myeloma (MM): Final Phase 1 Results and Phase 2 Data Richardson PG et al. Proc ASH 2013; Abstract 535.

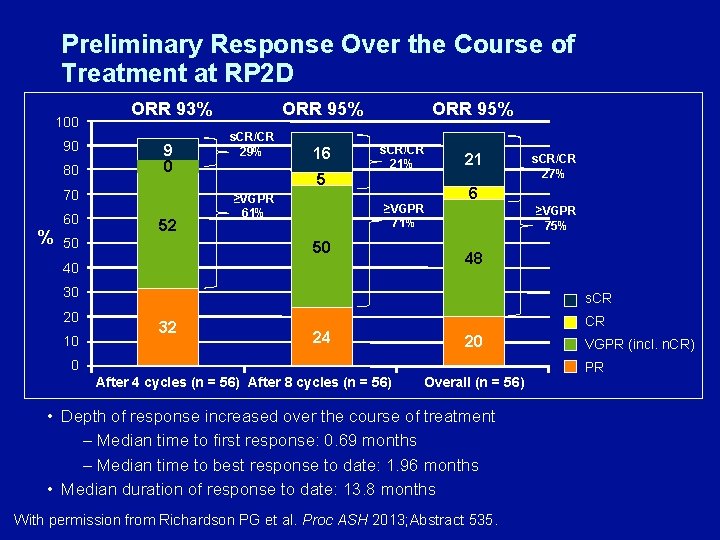
Preliminary Response Over the Course of Treatment at RP 2 D 100 90 80 ORR 93% 9 0 70 % 60 52 50 ORR 95% s. CR/CR 29% 16 5 ≥VGPR 61% ORR 95% s. CR/CR 21% 21 6 ≥VGPR 71% 50 40 ≥VGPR 75% 48 30 20 10 s. CR/CR 27% s. CR 32 24 CR 20 0 After 4 cycles (n = 56) After 8 cycles (n = 56) Overall (n = 56) • Depth of response increased over the course of treatment – Median time to first response: 0. 69 months – Median time to best response to date: 1. 96 months • Median duration of response to date: 13. 8 months With permission from Richardson PG et al. Proc ASH 2013; Abstract 535. VGPR (incl. n. CR) PR

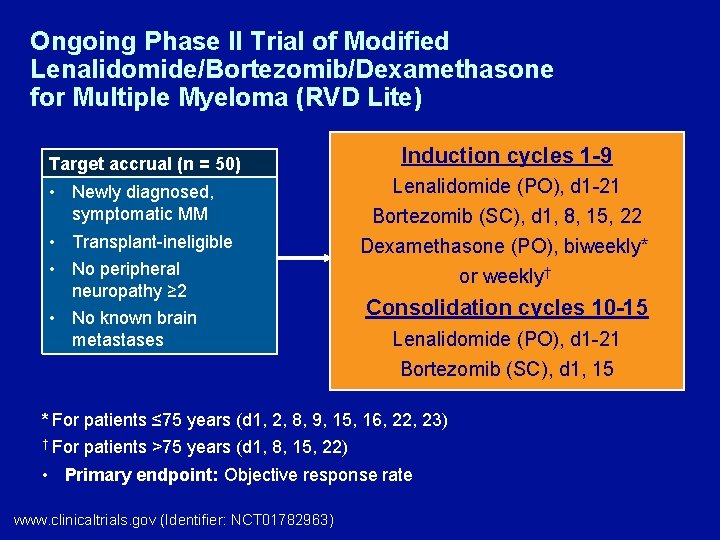
Ongoing Phase II Trial of Modified Lenalidomide/Bortezomib/Dexamethasone for Multiple Myeloma (RVD Lite) Target accrual (n = 50) • Newly diagnosed, symptomatic MM • Transplant-ineligible • No peripheral neuropathy ≥ 2 • No known brain metastases Induction cycles 1 -9 Lenalidomide (PO), d 1 -21 Bortezomib (SC), d 1, 8, 15, 22 Dexamethasone (PO), biweekly* or weekly† Consolidation cycles 10 -15 Lenalidomide (PO), d 1 -21 Bortezomib (SC), d 1, 15 * For patients ≤ 75 years (d 1, 2, 8, 9, 15, 16, 22, 23) † For patients >75 years (d 1, 8, 15, 22) • Primary endpoint: Objective response rate www. clinicaltrials. gov (Identifier: NCT 01782963)

Initial Phase 3 Results of the FIRST (Frontline Investigation of Lenalidomide + Dexamethasone versus Standard Thalidomide) Trial (MM-020/IFM 07 01) in Newly Diagnosed Multiple Myeloma (NDMM) Patients (Pts) Ineligible for Stem Cell Transplantation (SCT) Facon T et al. Proc ASH 2013; Abstract 2.

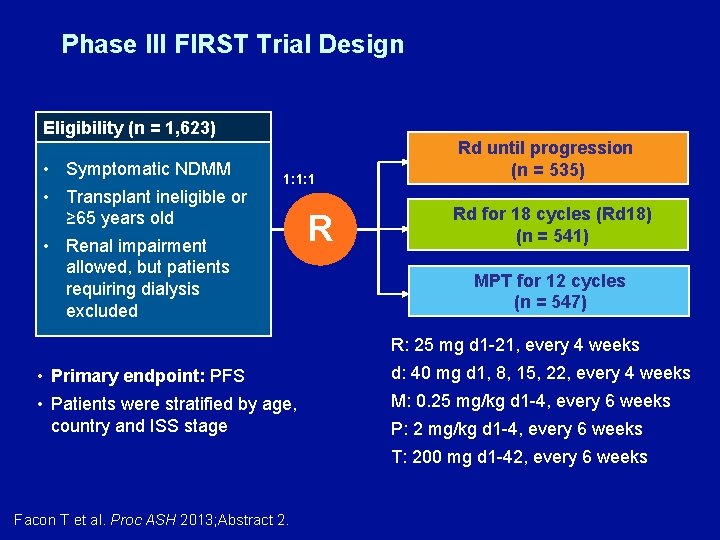
Phase III FIRST Trial Design Eligibility (n = 1, 623) • Symptomatic NDMM • Transplant ineligible or ≥ 65 years old • Renal impairment allowed, but patients requiring dialysis excluded 1: 1: 1 R Rd until progression (n = 535) Rd for 18 cycles (Rd 18) (n = 541) MPT for 12 cycles (n = 547) R: 25 mg d 1 -21, every 4 weeks • Primary endpoint: PFS d: 40 mg d 1, 8, 15, 22, every 4 weeks • Patients were stratified by age, country and ISS stage M: 0. 25 mg/kg d 1 -4, every 6 weeks P: 2 mg/kg d 1 -4, every 6 weeks T: 200 mg d 1 -42, every 6 weeks Facon T et al. Proc ASH 2013; Abstract 2.

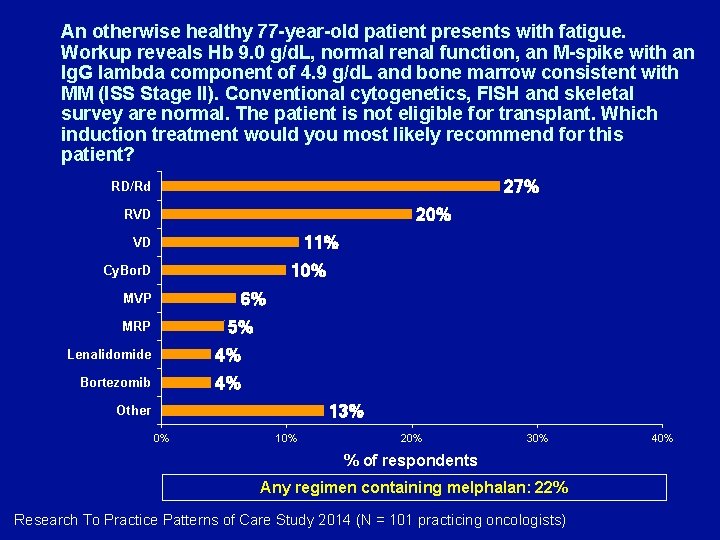
An otherwise healthy 77 -year-old patient presents with fatigue. Workup reveals Hb 9. 0 g/d. L, normal renal function, an M-spike with an Ig. G lambda component of 4. 9 g/d. L and bone marrow consistent with MM (ISS Stage II). Conventional cytogenetics, FISH and skeletal survey are normal. The patient is not eligible for transplant. Which induction treatment would you most likely recommend for this patient? 27% RD/Rd 20% RVD 11% VD 10% Cy. Bor. D 6% MVP 5% MRP Lenalidomide 4% Bortezomib 4% 13% Other 0% 10% 20% 30% % of respondents Any regimen containing melphalan: 22% Research To Practice Patterns of Care Study 2014 (N = 101 practicing oncologists) 40%

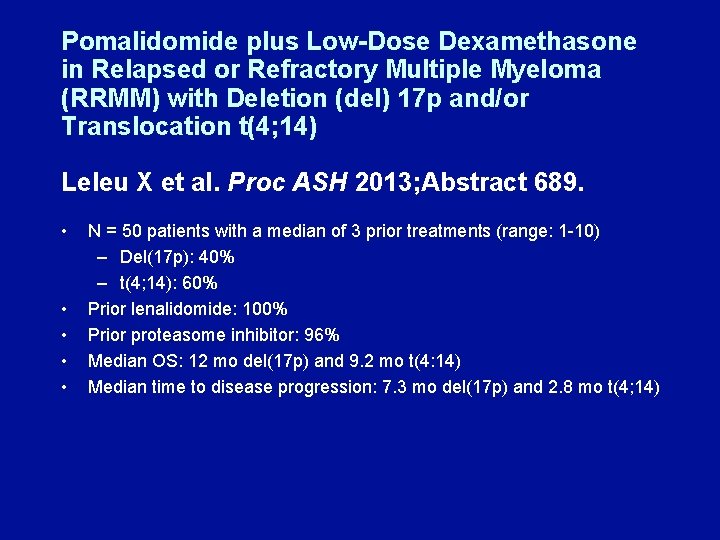
Pomalidomide plus Low-Dose Dexamethasone in Relapsed or Refractory Multiple Myeloma (RRMM) with Deletion (del) 17 p and/or Translocation t(4; 14) Leleu X et al. Proc ASH 2013; Abstract 689. • • • N = 50 patients with a median of 3 prior treatments (range: 1 -10) – Del(17 p): 40% – t(4; 14): 60% Prior lenalidomide: 100% Prior proteasome inhibitor: 96% Median OS: 12 mo del(17 p) and 9. 2 mo t(4: 14) Median time to disease progression: 7. 3 mo del(17 p) and 2. 8 mo t(4; 14)

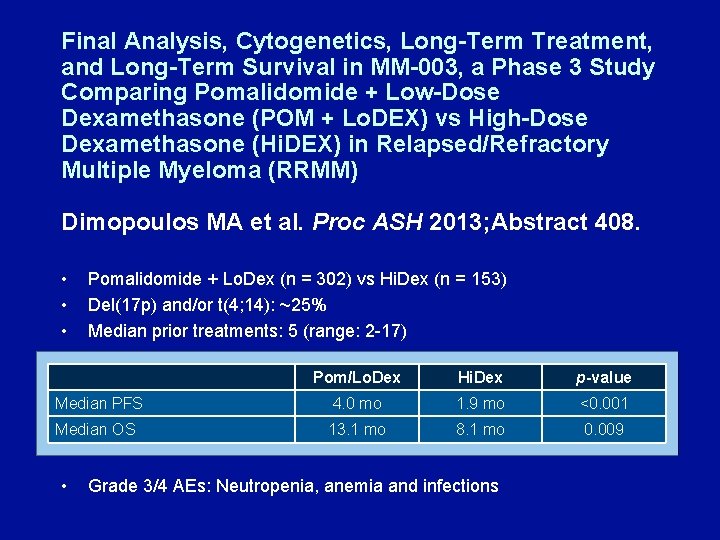
Final Analysis, Cytogenetics, Long-Term Treatment, and Long-Term Survival in MM-003, a Phase 3 Study Comparing Pomalidomide + Low-Dose Dexamethasone (POM + Lo. DEX) vs High-Dose Dexamethasone (Hi. DEX) in Relapsed/Refractory Multiple Myeloma (RRMM) Dimopoulos MA et al. Proc ASH 2013; Abstract 408. • • • Pomalidomide + Lo. Dex (n = 302) vs Hi. Dex (n = 153) Del(17 p) and/or t(4; 14): ~25% Median prior treatments: 5 (range: 2 -17) Pom/Lo. Dex Hi. Dex p-value Median PFS 4. 0 mo 1. 9 mo <0. 001 Median OS 13. 1 mo 8. 1 mo 0. 009 • Grade 3/4 AEs: Neutropenia, anemia and infections

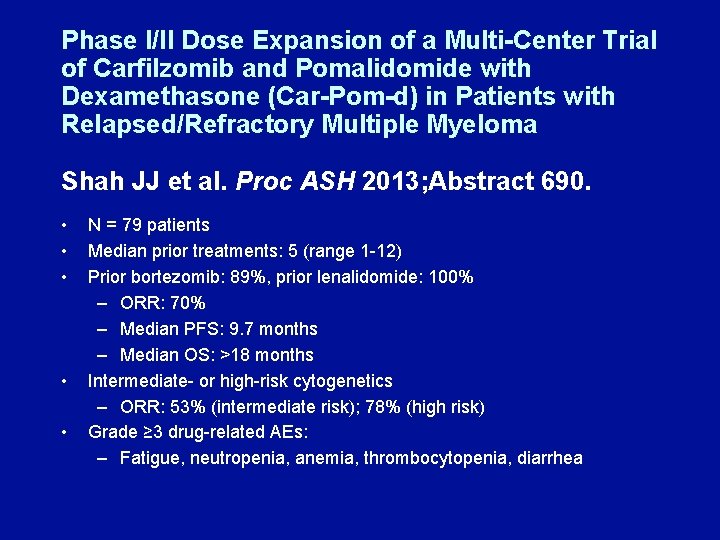
Phase I/II Dose Expansion of a Multi-Center Trial of Carfilzomib and Pomalidomide with Dexamethasone (Car-Pom-d) in Patients with Relapsed/Refractory Multiple Myeloma Shah JJ et al. Proc ASH 2013; Abstract 690. • • • N = 79 patients Median prior treatments: 5 (range 1 -12) Prior bortezomib: 89%, prior lenalidomide: 100% – ORR: 70% – Median PFS: 9. 7 months – Median OS: >18 months Intermediate- or high-risk cytogenetics – ORR: 53% (intermediate risk); 78% (high risk) Grade ≥ 3 drug-related AEs: – Fatigue, neutropenia, anemia, thrombocytopenia, diarrhea


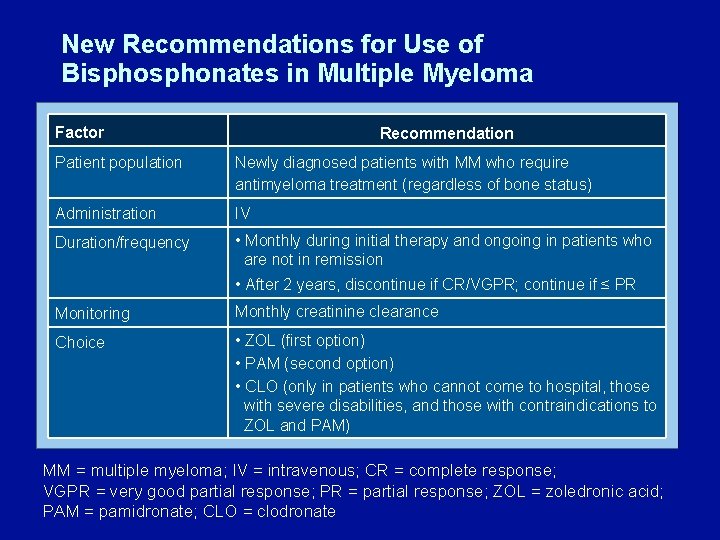
New Recommendations for Use of Bisphonates in Multiple Myeloma Factor Recommendation Patient population Newly diagnosed patients with MM who require antimyeloma treatment (regardless of bone status) Administration IV Duration/frequency • Monthly during initial therapy and ongoing in patients who are not in remission • After 2 years, discontinue if CR/VGPR; continue if ≤ PR Monitoring Monthly creatinine clearance Choice • ZOL (first option) • PAM (second option) • CLO (only in patients who cannot come to hospital, those with severe disabilities, and those with contraindications to ZOL and PAM) MM = multiple myeloma; IV = intravenous; CR = complete response; VGPR = very good partial response; PR = partial response; ZOL = zoledronic acid; PAM = pamidronate; CLO = clodronate


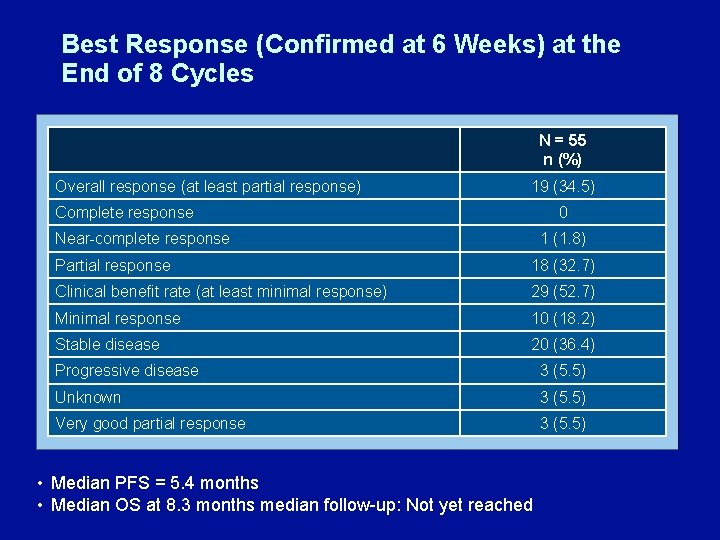
Best Response (Confirmed at 6 Weeks) at the End of 8 Cycles N = 55 n (%) Overall response (at least partial response) 19 (34. 5) Complete response 0 Near-complete response 1 (1. 8) Partial response 18 (32. 7) Clinical benefit rate (at least minimal response) 29 (52. 7) Minimal response 10 (18. 2) Stable disease 20 (36. 4) Progressive disease 3 (5. 5) Unknown 3 (5. 5) Very good partial response 3 (5. 5) • Median PFS = 5. 4 months • Median OS at 8. 3 months median follow-up: Not yet reached

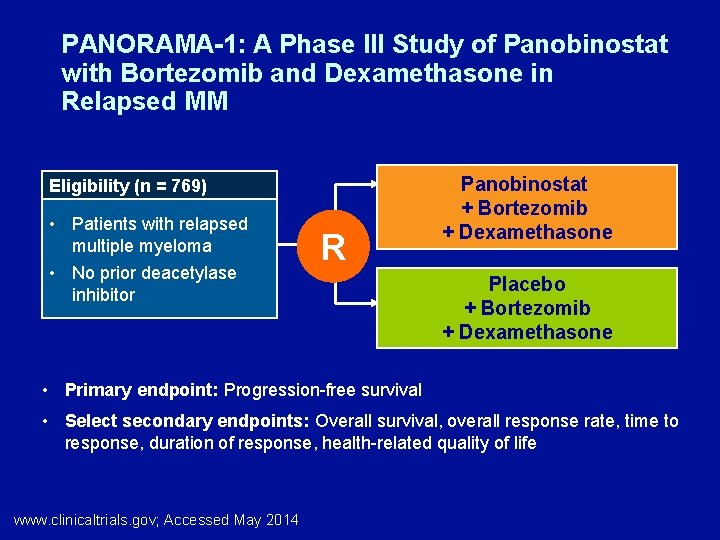
PANORAMA-1: A Phase III Study of Panobinostat with Bortezomib and Dexamethasone in Relapsed MM Eligibility (n = 769) • Patients with relapsed multiple myeloma • No prior deacetylase inhibitor R Panobinostat + Bortezomib + Dexamethasone Placebo + Bortezomib + Dexamethasone • Primary endpoint: Progression-free survival • Select secondary endpoints: Overall survival, overall response rate, time to response, duration of response, health-related quality of life www. clinicaltrials. gov; Accessed May 2014

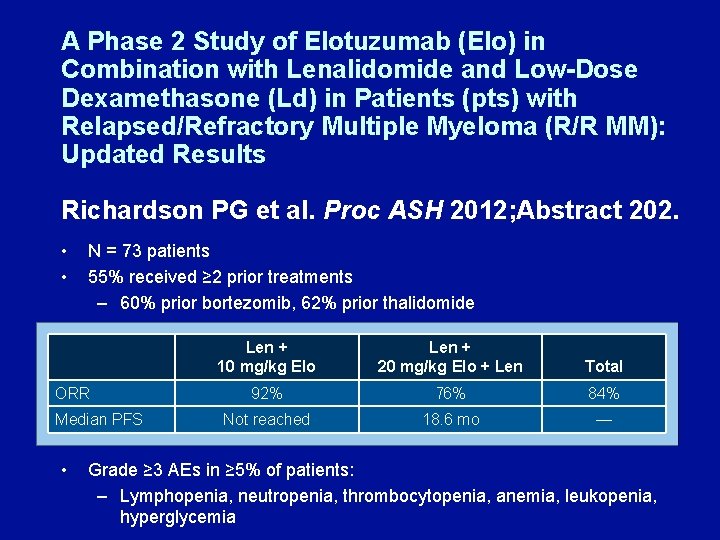
A Phase 2 Study of Elotuzumab (Elo) in Combination with Lenalidomide and Low-Dose Dexamethasone (Ld) in Patients (pts) with Relapsed/Refractory Multiple Myeloma (R/R MM): Updated Results Richardson PG et al. Proc ASH 2012; Abstract 202. • • N = 73 patients 55% received ≥ 2 prior treatments – 60% prior bortezomib, 62% prior thalidomide ORR Median PFS • Len + 10 mg/kg Elo Len + 20 mg/kg Elo + Len Total 92% 76% 84% Not reached 18. 6 mo — Grade ≥ 3 AEs in ≥ 5% of patients: – Lymphopenia, neutropenia, thrombocytopenia, anemia, leukopenia, hyperglycemia

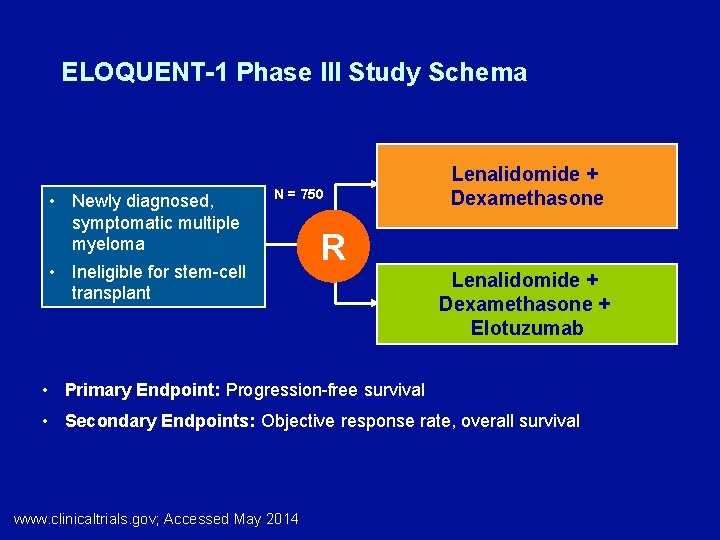
ELOQUENT-1 Phase III Study Schema • Newly diagnosed, symptomatic multiple myeloma • Ineligible for stem-cell transplant N = 750 Lenalidomide + Dexamethasone R Lenalidomide + Dexamethasone + Elotuzumab • Primary Endpoint: Progression-free survival • Secondary Endpoints: Objective response rate, overall survival www. clinicaltrials. gov; Accessed May 2014

Carfilzomib, Rituximab and Dexamethasone (Ca. RD) Is Highly Active and Offers a Neuropathy Sparing Approach for Proteasome. Inhibitor Based Therapy in Waldenström’s Macroglobulinemia Treon SP et al. Proc ASH 2013; Abstract 757.

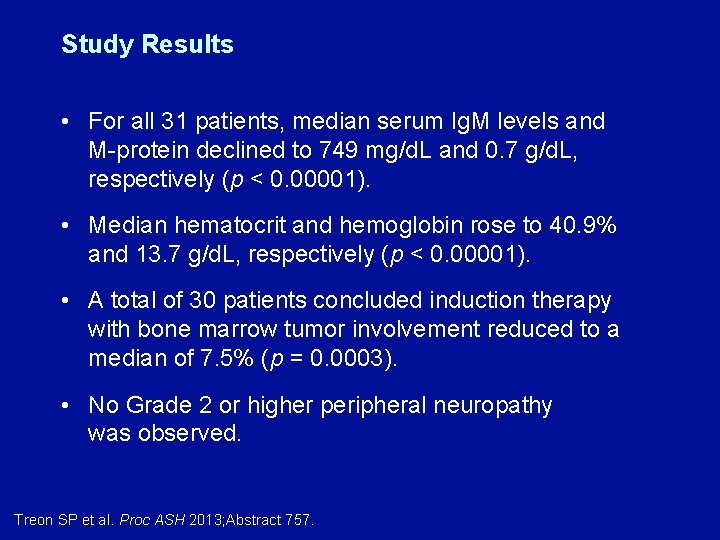
Study Results • For all 31 patients, median serum Ig. M levels and M-protein declined to 749 mg/d. L and 0. 7 g/d. L, respectively (p < 0. 00001). • Median hematocrit and hemoglobin rose to 40. 9% and 13. 7 g/d. L, respectively (p < 0. 00001). • A total of 30 patients concluded induction therapy with bone marrow tumor involvement reduced to a median of 7. 5% (p = 0. 0003). • No Grade 2 or higher peripheral neuropathy was observed. Treon SP et al. Proc ASH 2013; Abstract 757.

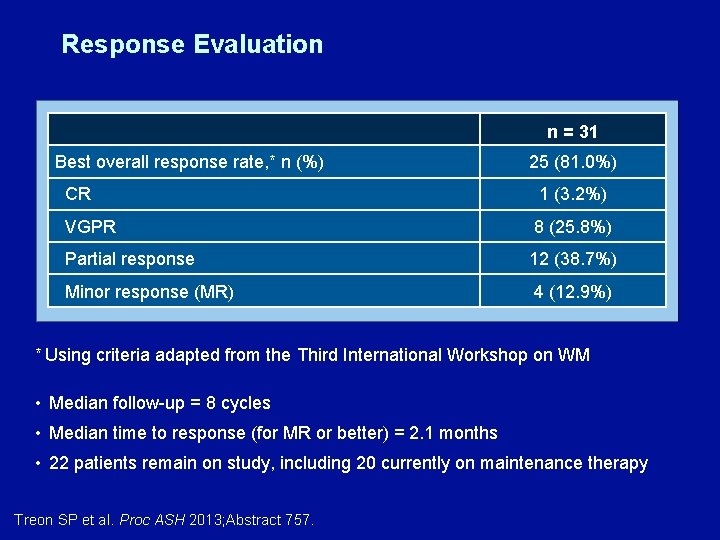
Response Evaluation n = 31 Best overall response rate, * n (%) 25 (81. 0%) CR 1 (3. 2%) VGPR 8 (25. 8%) Partial response 12 (38. 7%) Minor response (MR) 4 (12. 9%) * Using criteria adapted from the Third International Workshop on WM • Median follow-up = 8 cycles • Median time to response (for MR or better) = 2. 1 months • 22 patients remain on study, including 20 currently on maintenance therapy Treon SP et al. Proc ASH 2013; Abstract 757.

Treon SP et al. NEJM 2012; 367(9): 826 -33.

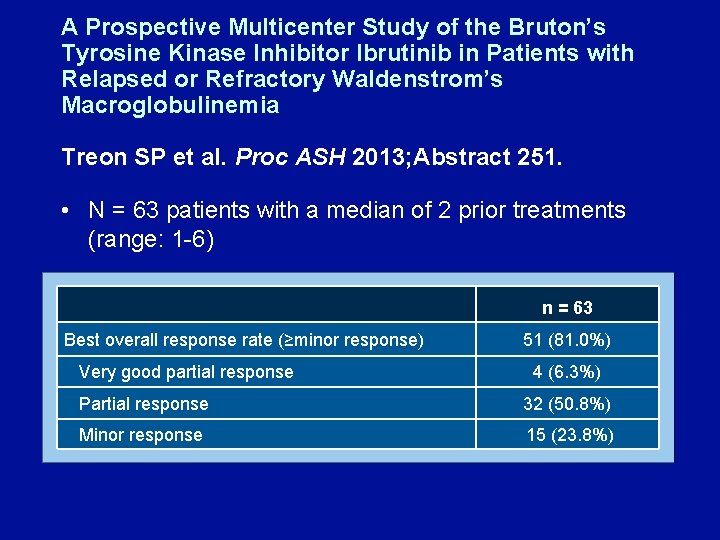
A Prospective Multicenter Study of the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib in Patients with Relapsed or Refractory Waldenstrom’s Macroglobulinemia Treon SP et al. Proc ASH 2013; Abstract 251. • N = 63 patients with a median of 2 prior treatments (range: 1 -6) n = 63 Best overall response rate (≥minor response) Very good partial response 51 (81. 0%) 4 (6. 3%) Partial response 32 (50. 8%) Minor response 15 (23. 8%)

Lohr JG et al. Cancer Cell 2014; 25(1): 91 -101.

Clinical and Correlative Pilot Study of Carfilzomib, Lenalidomide, and Dexamethasone Followed by Lenalidomide Extended Dosing (CRd–R) in High Risk Smoldering Multiple Myeloma Patients Landgren O et al. Proc ASH 2013; Abstract 1939.