RTEC A WEEK 4 GENERAL SCIENCE REVIEW XRAY






- Slides: 47

RTEC A - WEEK 4 GENERAL SCIENCE REVIEW & X-RAY PRODUCTION IN THE TUBE ***FINAL***

ALERT Please do not print the lecture until you see “FINAL” on the first slide.

Objectives • General Science review • Atomic interactions in the tube

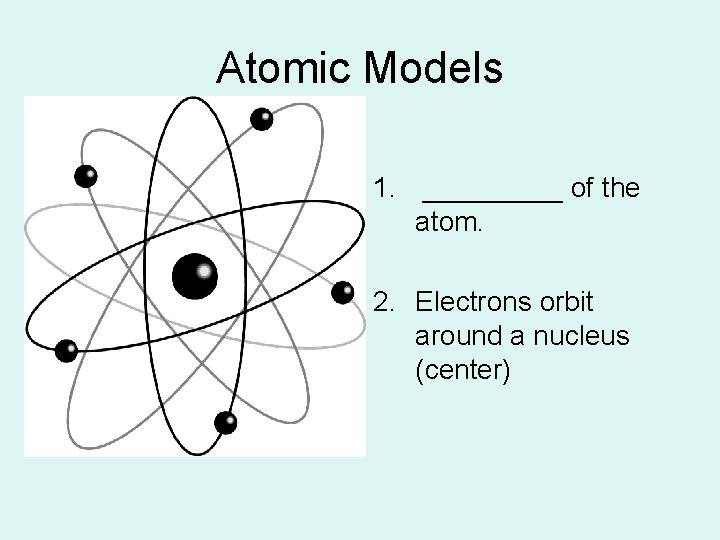
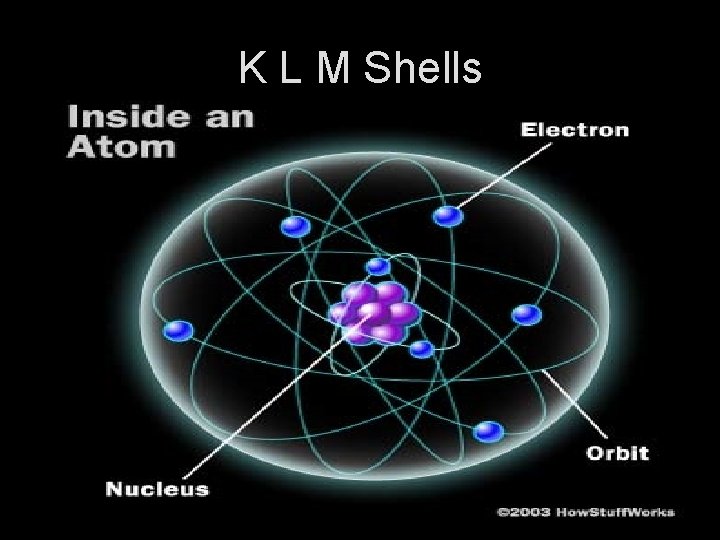
Atomic Models 1. _____ of the atom. 2. Electrons orbit around a nucleus (center)

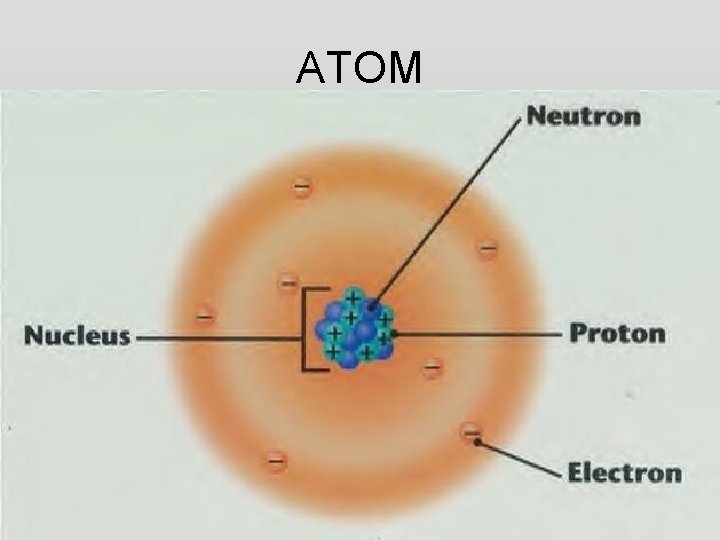
ATOM

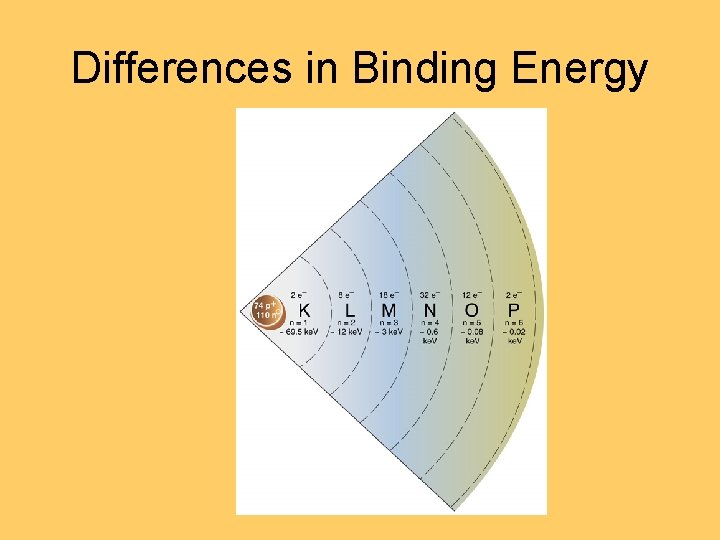
Differences in Binding Energy

K L M Shells

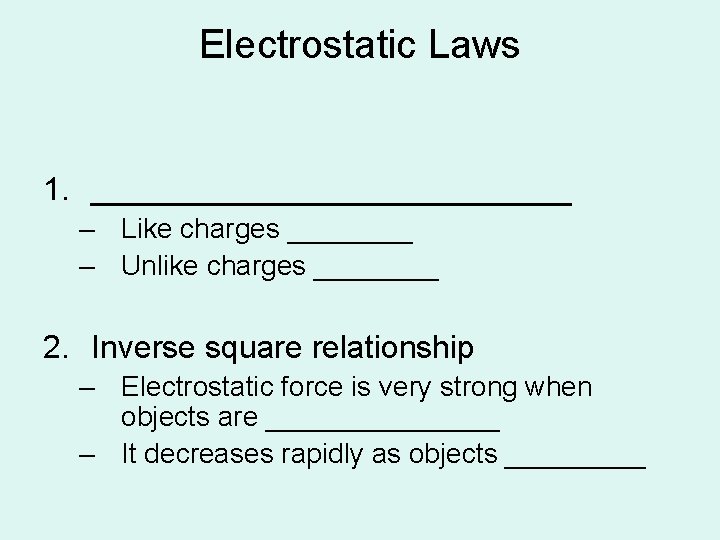
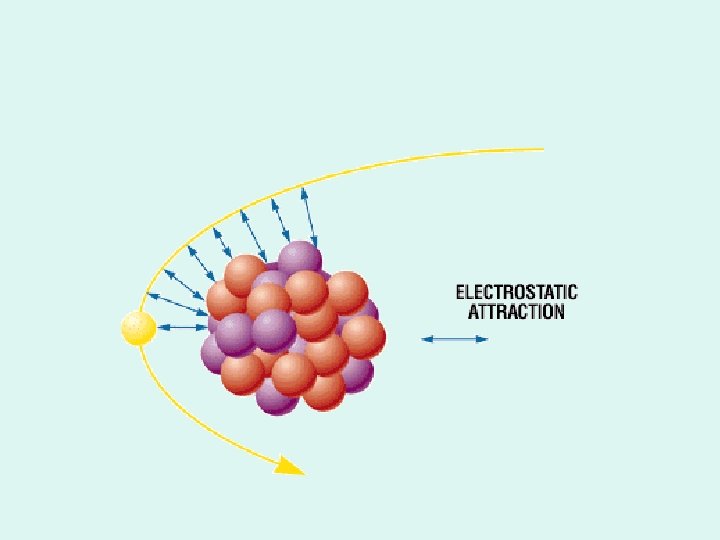
Electrostatic Laws 1. ______________ – Like charges ____ – Unlike charges ____ 2. Inverse square relationship – Electrostatic force is very strong when objects are ________ – It decreases rapidly as objects _____

How “X-rays” are created TO PRODUCE X-RAYS YOU NEED: 1. _______________ 2. ______________ 3. _______________

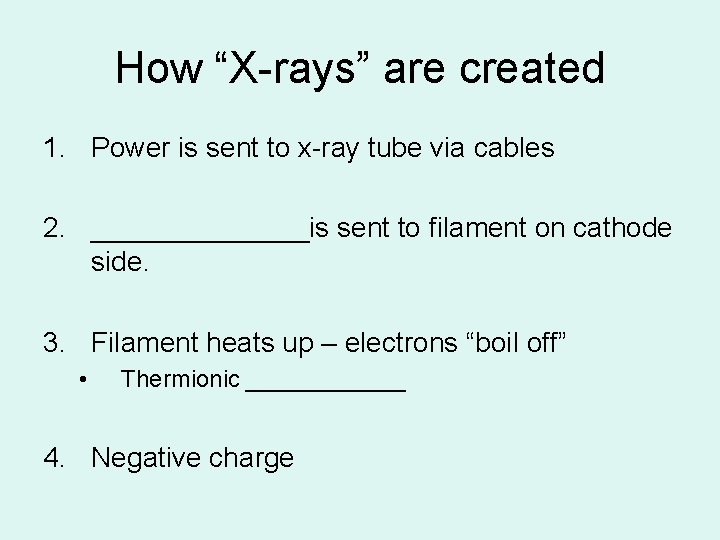
How “X-rays” are created 1. Power is sent to x-ray tube via cables 2. _______is sent to filament on cathode side. 3. Filament heats up – electrons “boil off” • Thermionic ______ 4. Negative charge

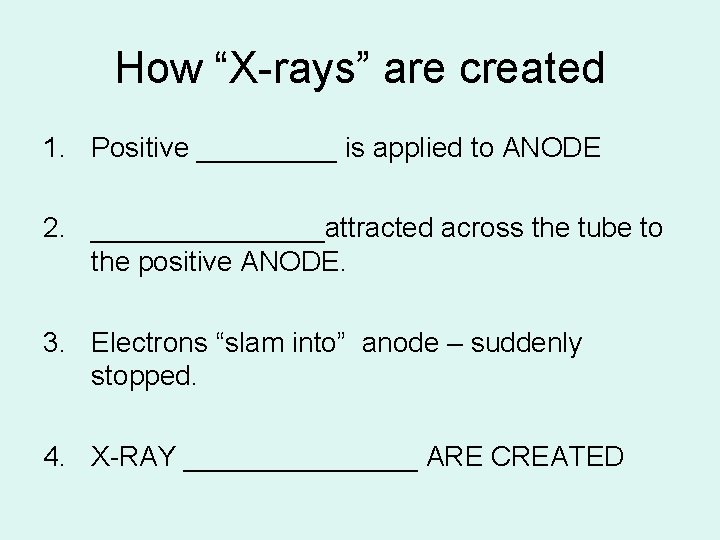
How “X-rays” are created 1. Positive _____ is applied to ANODE 2. ________attracted across the tube to the positive ANODE. 3. Electrons “slam into” anode – suddenly stopped. 4. X-RAY ________ ARE CREATED

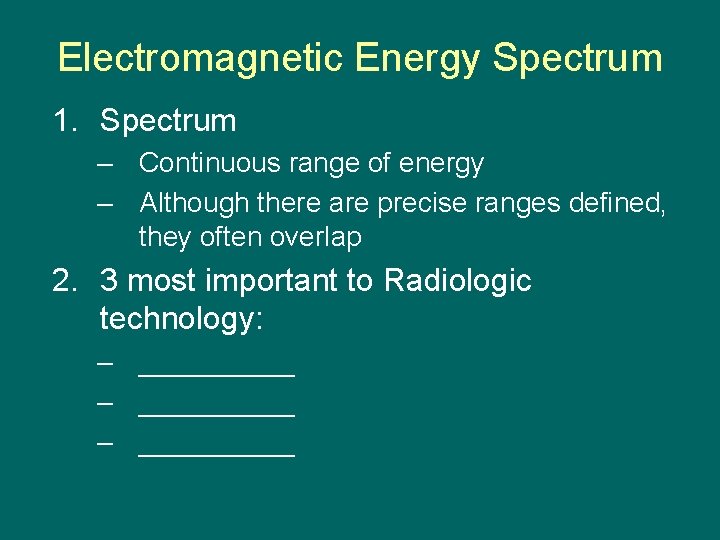
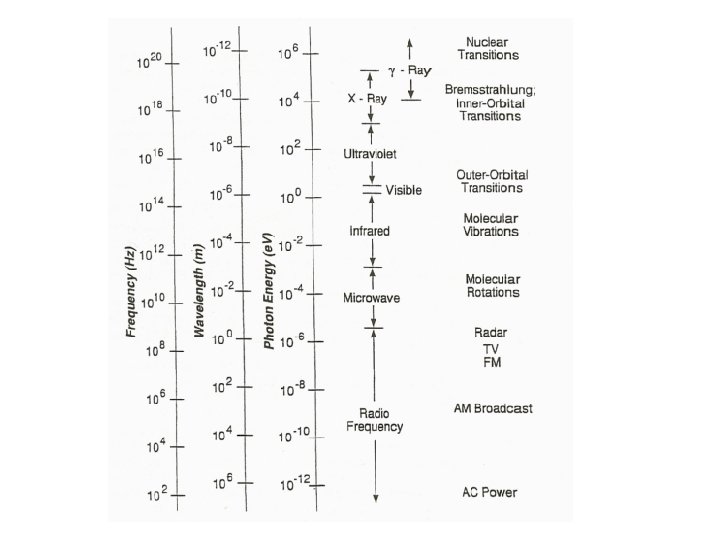
Electromagnetic Energy Spectrum 1. Spectrum – Continuous range of energy – Although there are precise ranges defined, they often overlap 2. 3 most important to Radiologic technology: – __________ – _____

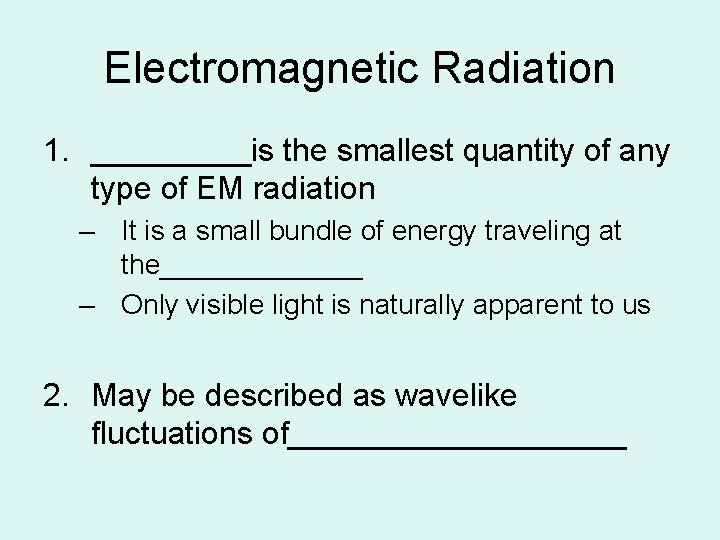
Electromagnetic Radiation 1. _____is the smallest quantity of any type of EM radiation – It is a small bundle of energy traveling at the_______ – Only visible light is naturally apparent to us 2. May be described as wavelike fluctuations of__________

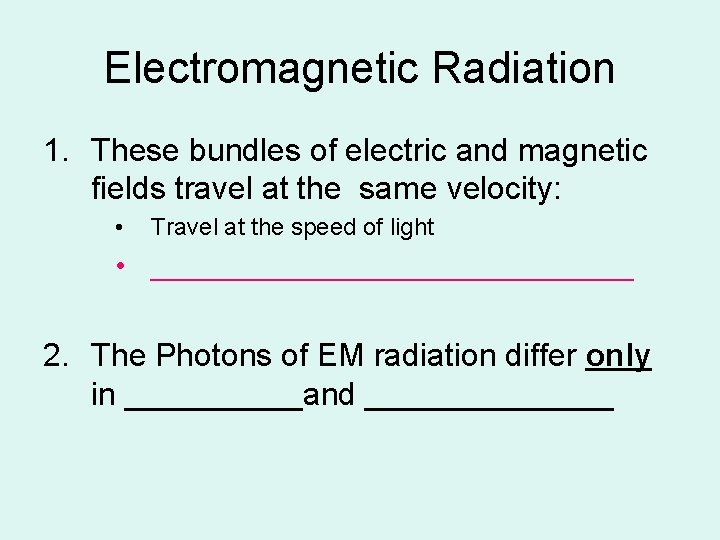
Electromagnetic Radiation 1. These bundles of electric and magnetic fields travel at the same velocity: • Travel at the speed of light • ________________ 2. The Photons of EM radiation differ only in _____and _______

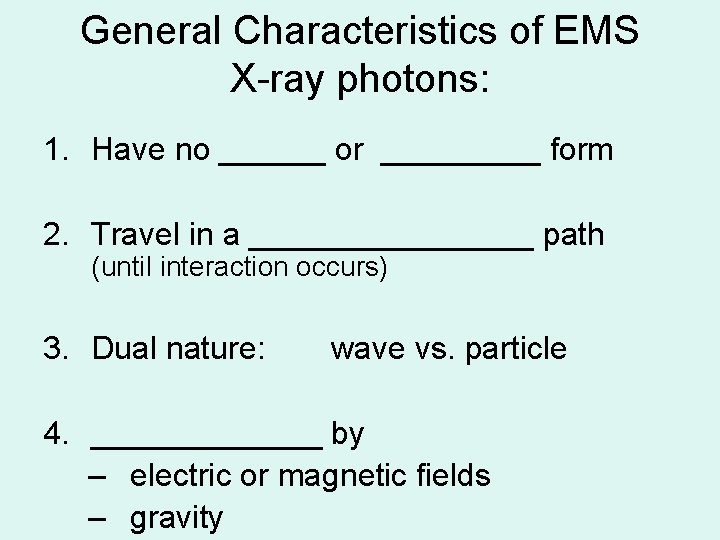
General Characteristics of EMS X-ray photons: 1. Have no ______ or _____ form 2. Travel in a ________ path (until interaction occurs) 3. Dual nature: wave vs. particle 4. _______ by – electric or magnetic fields – gravity

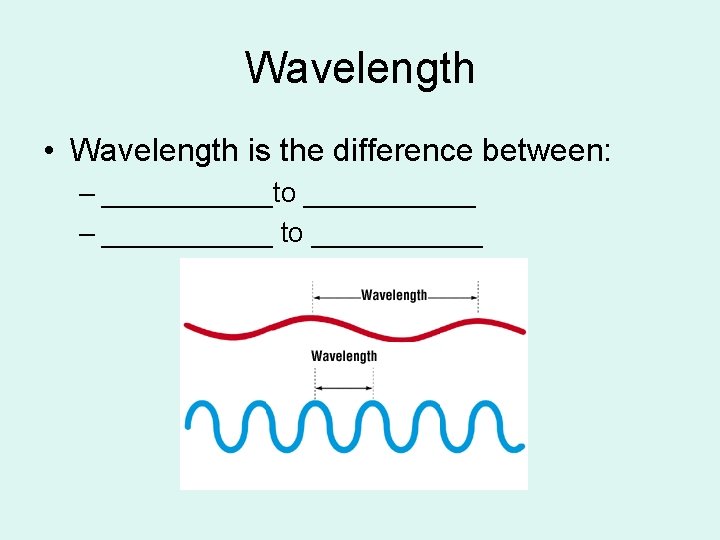
Wavelength • Wavelength is the difference between: – ______to ______ – ______ to ______

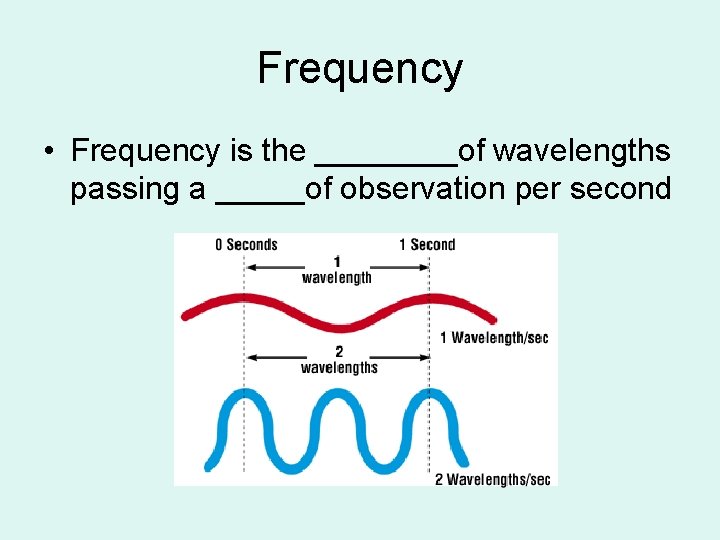
Frequency • Frequency is the ____of wavelengths passing a _____of observation per second

Wavelength and Frequency 1. Wavelength & frequency are inversely proportional – As Wavelength ____ frequency ______ – As wavelength _____ frequency ______ 2. Frequency and wavelength are closely associated with the relative energy of electromagnetic radiations. 3. More energetic radiations have _____ wavelengths and ____frequency.

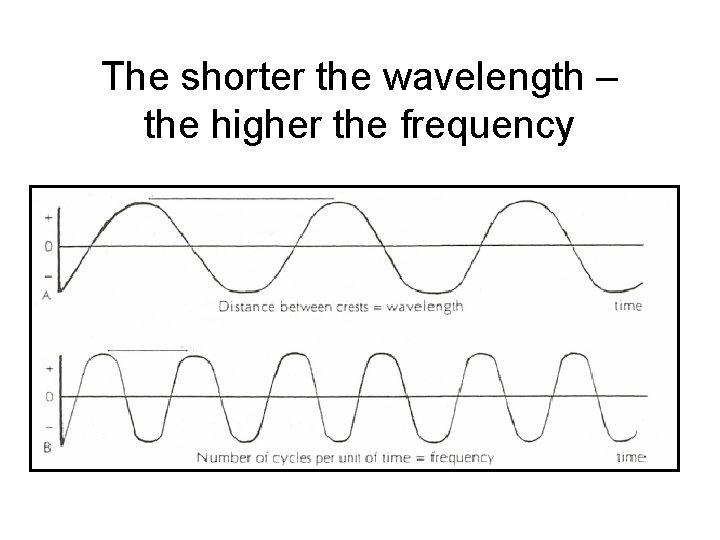
The shorter the wavelength – the higher the frequency


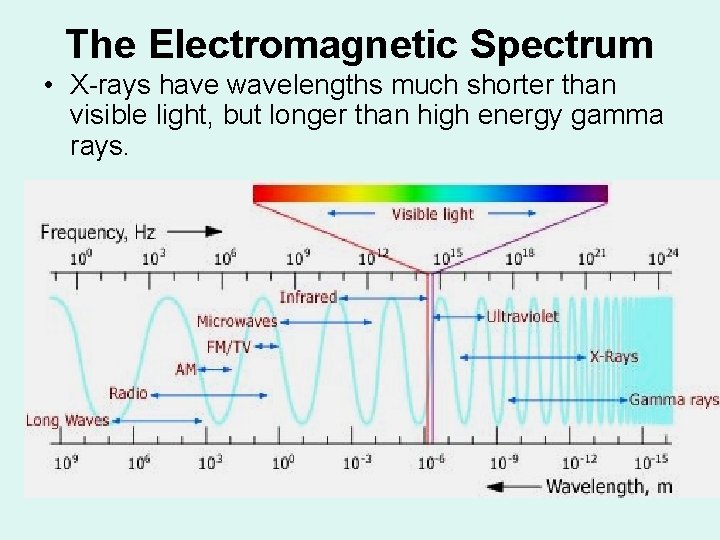
The Electromagnetic Spectrum • X-rays have wavelengths much shorter than visible light, but longer than high energy gamma rays.

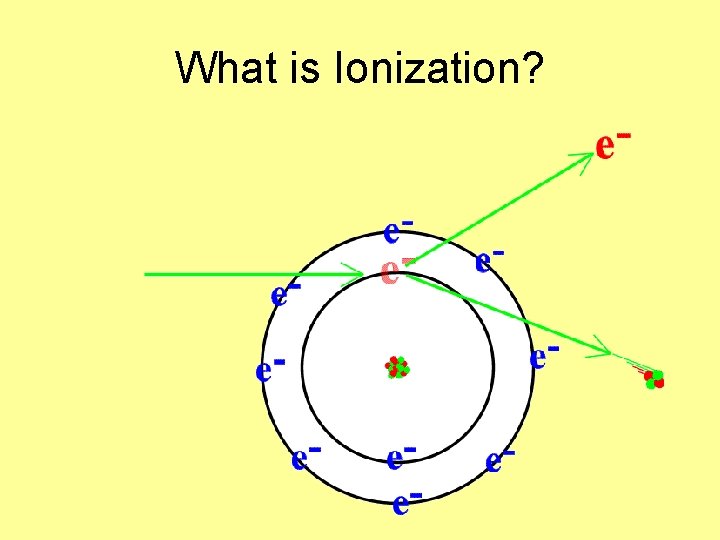
What is Ionization?

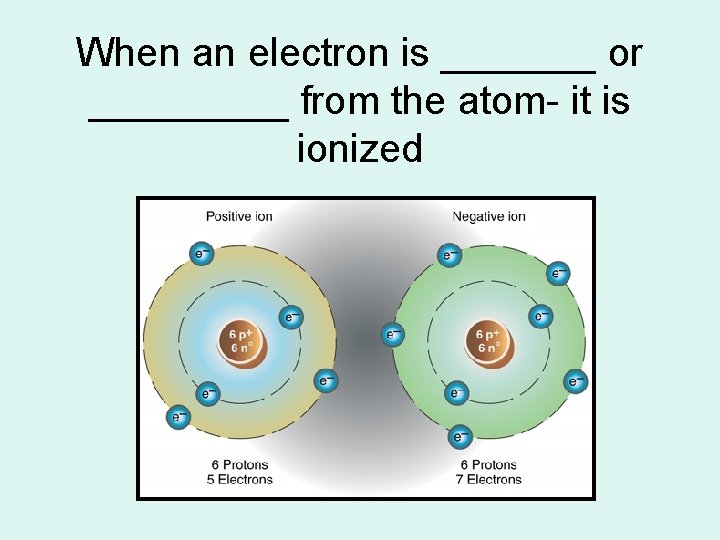
When an electron is _______ or _____ from the atom- it is ionized

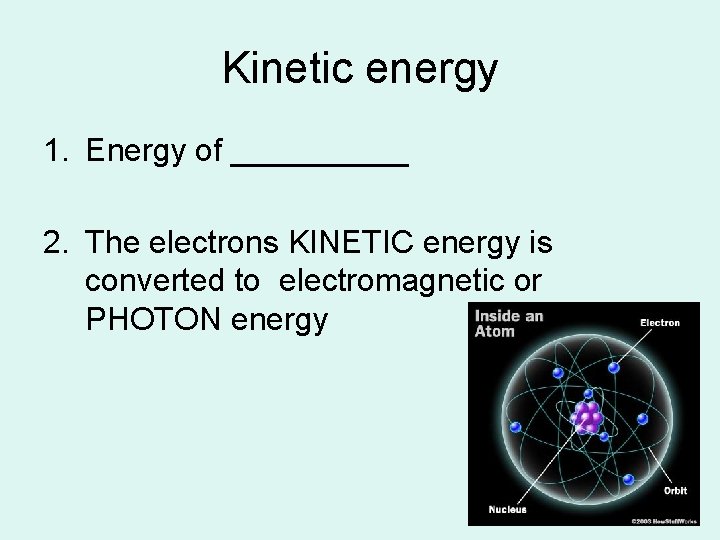
Kinetic energy 1. Energy of _____ 2. The electrons KINETIC energy is converted to electromagnetic or PHOTON energy

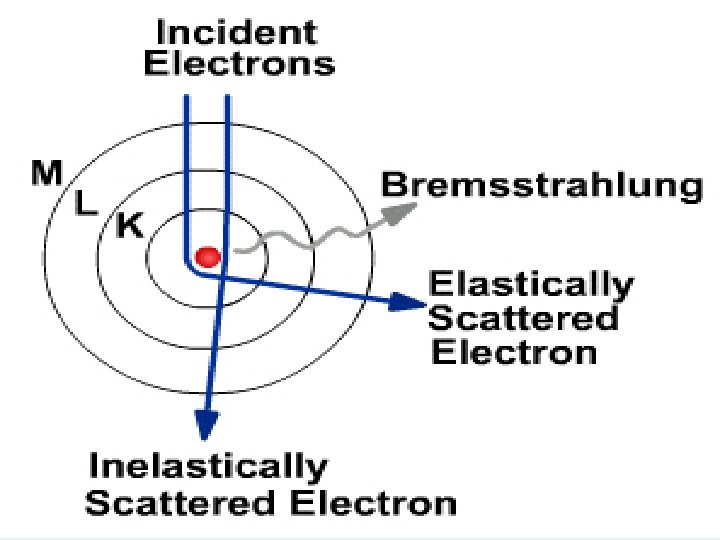
X-ray production begins at the atomic level Energy (photons) are released when the electron collides with another electron, or passes close to the nucleus of the atom – the change in energy of the shells –produces photons

X-ray Production in the TUBE

INTERACTIONS IN THE TUBE 1. ____________ 2. ____________ 3. ____________

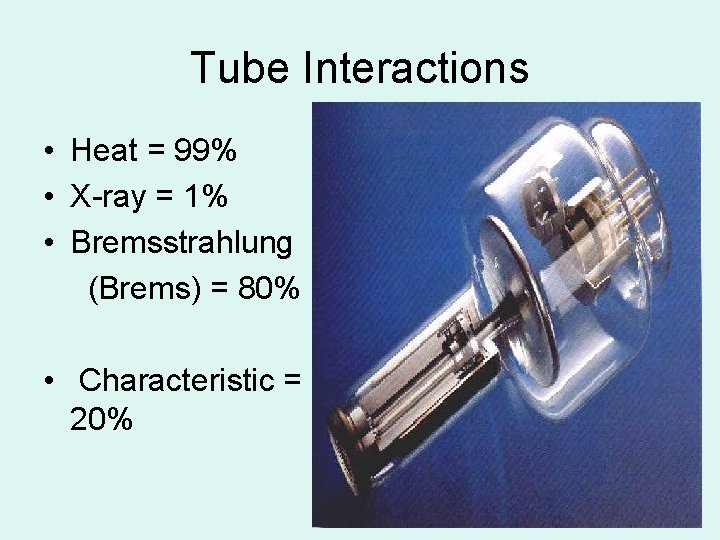
Tube Interactions • Heat = 99% • X-ray = 1% • Bremsstrahlung (Brems) = 80% • Characteristic = 20%

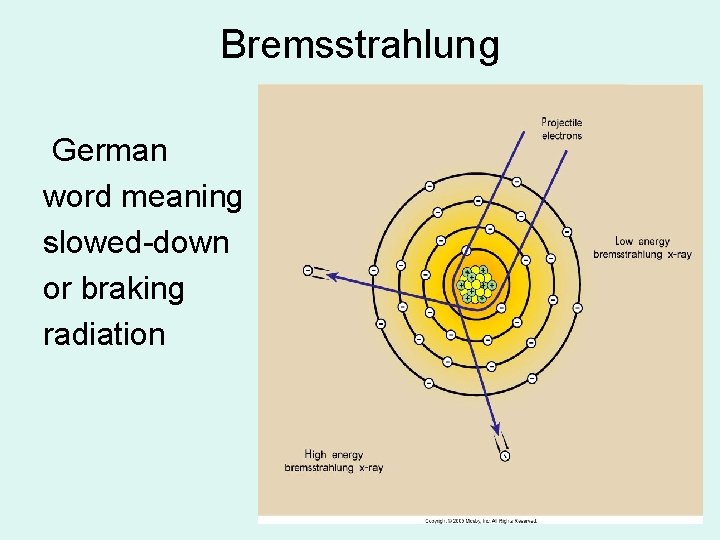
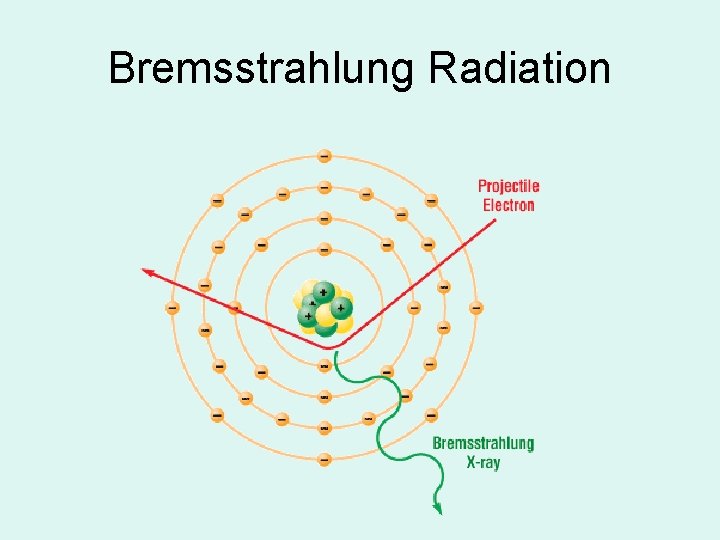
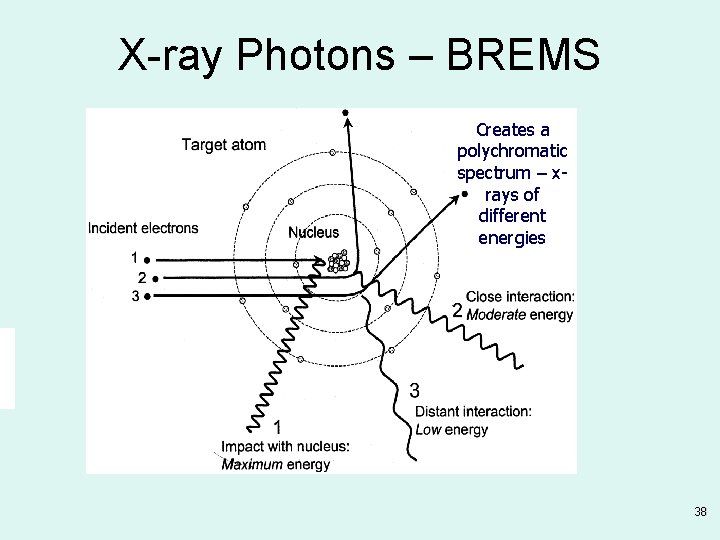
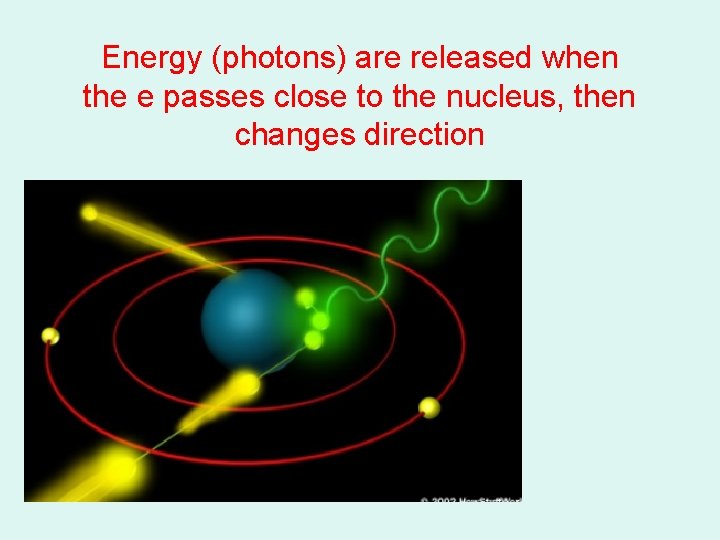
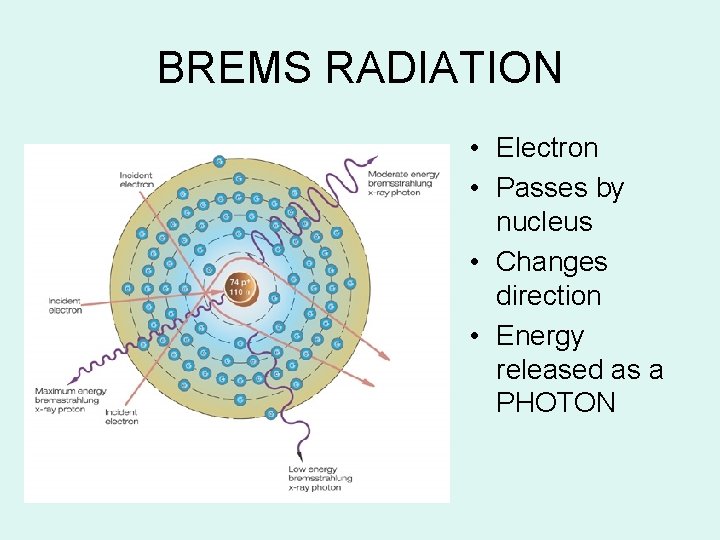
Bremsstrahlung Radiation • Heat & Characteristic produces EM energy by e- interacting with tungsten atoms e- of the target material • Bremsstrahlung is produced by e- passing by closely with the nucleus of a target tungsten atom – the change in direction of the electron – releases a photon of energy

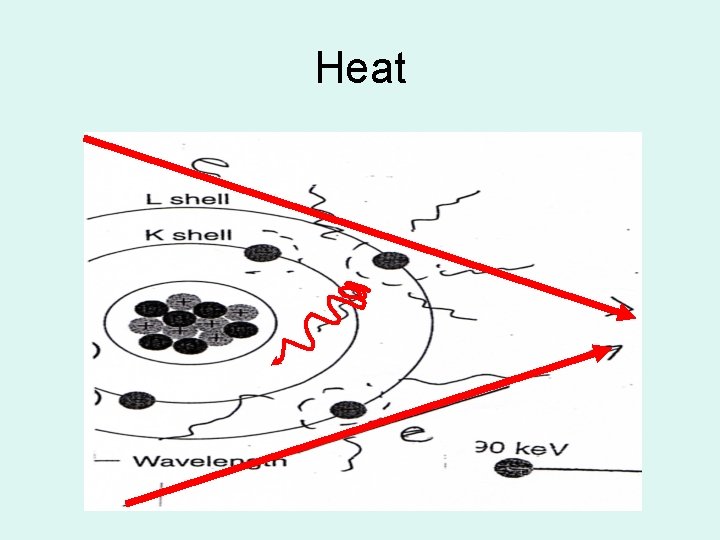
Heat • Most kinetic energy of projectile e- is converted into heat – 99% • Projectile e- interact with the outer-shell eof the target atoms but do not transfer enough energy to the outer-shell e- to ionize

Heat

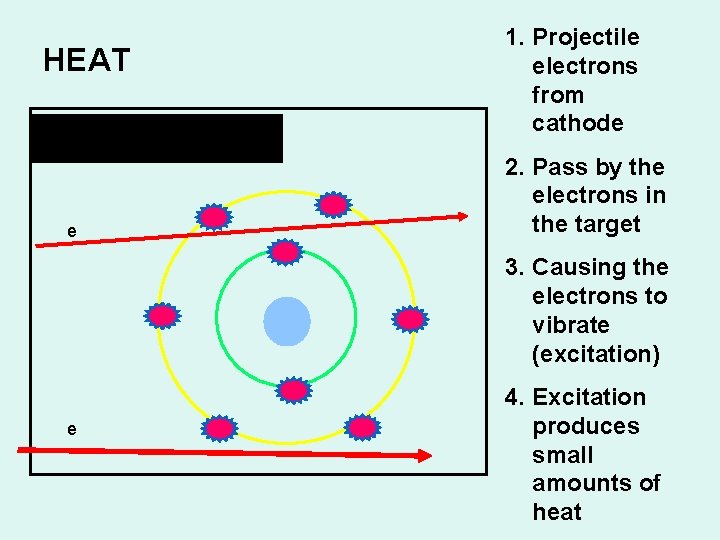
HEAT 8 p+ + 8 e- = neutral atom e 1. Projectile electrons from cathode 2. Pass by the electrons in the target 3. Causing the electrons to vibrate (excitation) e 4. Excitation produces small amounts of heat

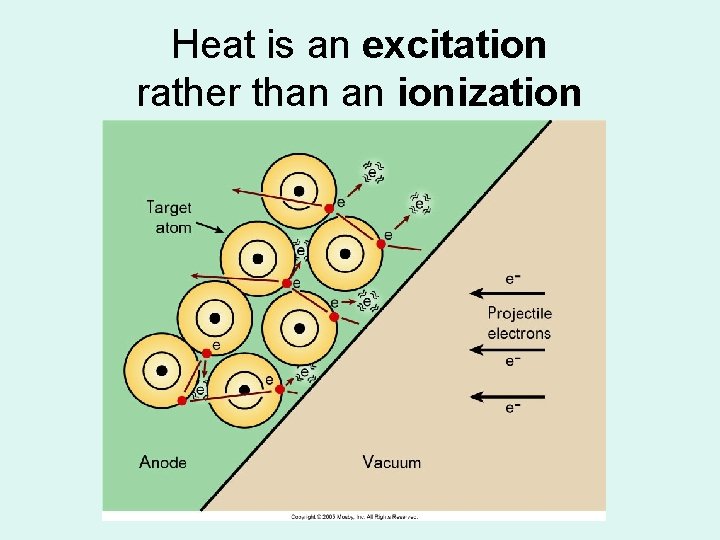
Heat is an excitation rather than an ionization

Bremsstrahlung German word meaning slowed-down or braking radiation



Bremsstrahlung Radiation

X-ray Photons – BREMS Creates a polychromatic spectrum – xrays of different energies 38

Energy (photons) are released when the e passes close to the nucleus, then changes direction

BREMS RADIATION • Electron • Passes by nucleus • Changes direction • Energy released as a PHOTON

Brem’s Radiation Animation • http: //www. coursewareobjects. com/objects /mrophysics_v 1/mod 08/0816 a. htm

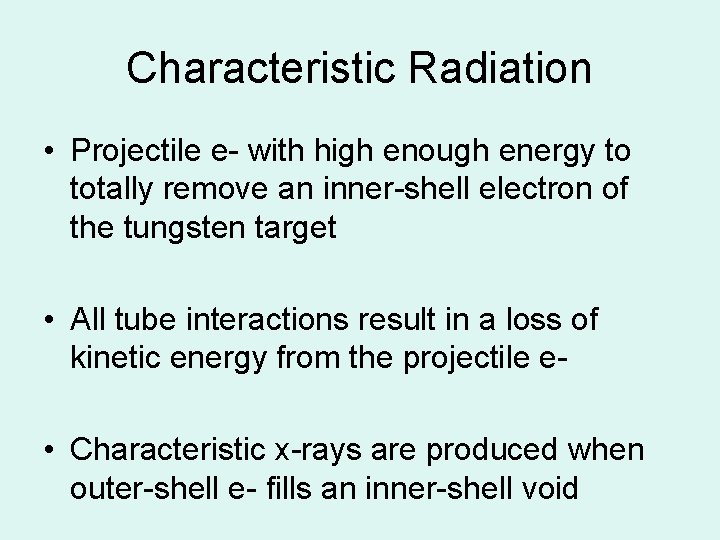
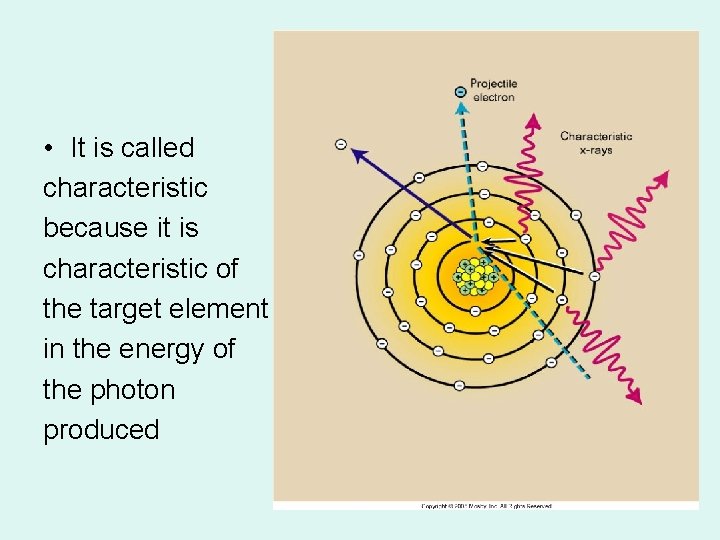
Characteristic Radiation • Projectile e- with high enough energy to totally remove an inner-shell electron of the tungsten target • All tube interactions result in a loss of kinetic energy from the projectile e • Characteristic x-rays are produced when outer-shell e- fills an inner-shell void

Characteristic Radiation (Tube)

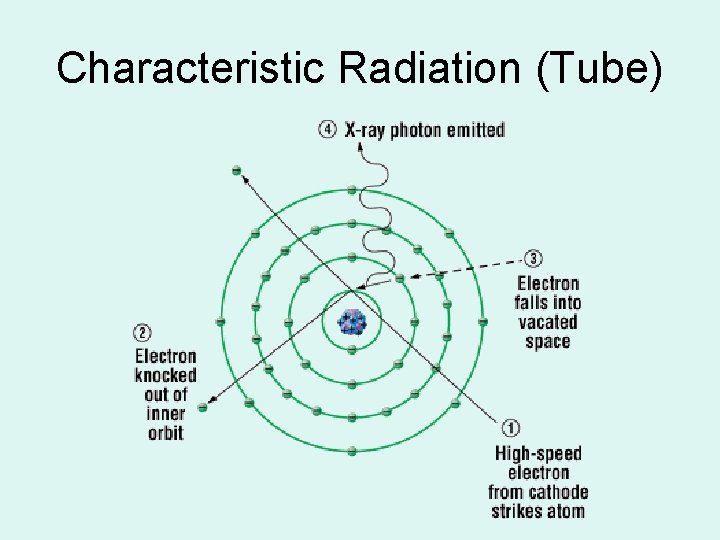
CHARACTERISTIC (in tube) 1. Electron hits ____ e in orbit – knocked out & creates a hole 2. Other E’s want to jump in 3. ____ released as PHOTONS

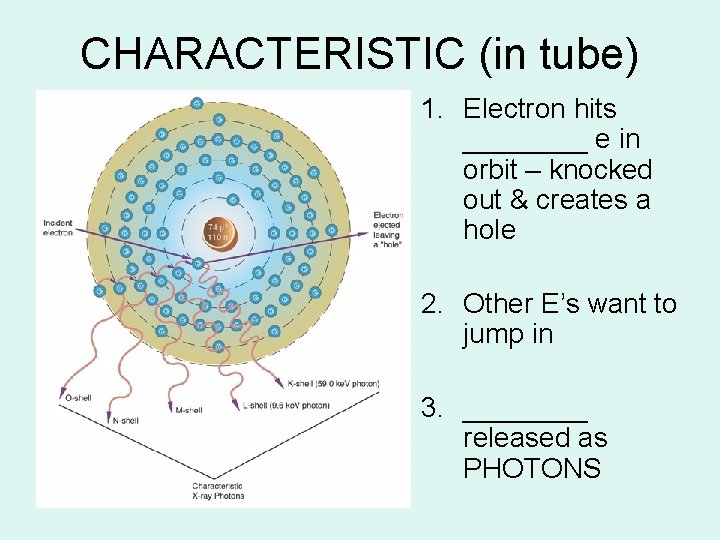
• It is called characteristic because it is characteristic of the target element in the energy of the photon produced

Characteristic Radiation Animation • http: //www. coursewareobjects. com/objects /mrophysics_v 1/mod 08/0808 a. htm

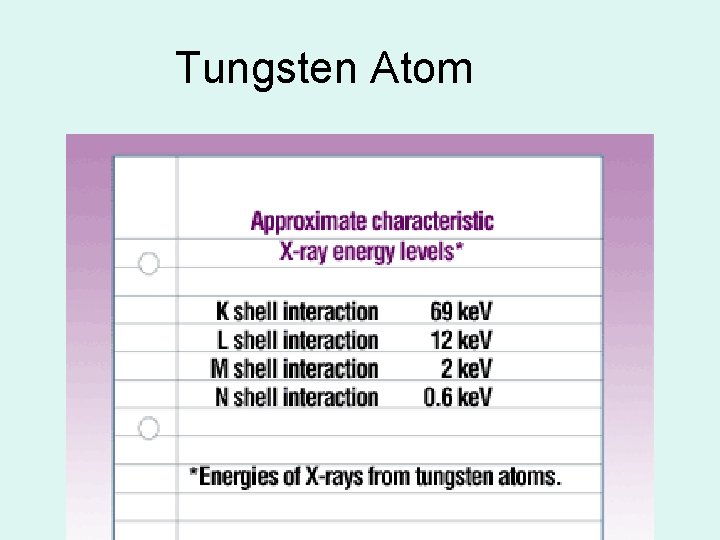
Tungsten Atom