Routine Immunization IMMUNISATION PROCESS OF ARTIFICIALLY PROVIDING IMMUNITY

































- Slides: 68

Routine Immunization

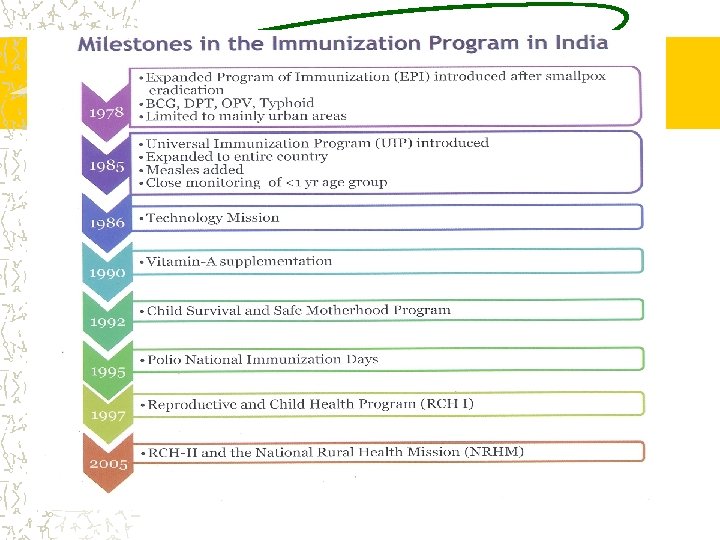
IMMUNISATION • PROCESS OF ARTIFICIALLY PROVIDING IMMUNITY • ACTIVE-VACCINES • LIVEATTENUATED, KILLED, TOXOID, SUBUNIT, RECOMBINANT • PASSIVE-IMMUNOGLOBULIN • MOST COST EFFECTIVE HEALTH CARE INTERVENTION AND BIRTH RIGHT OF EVERY CHILD


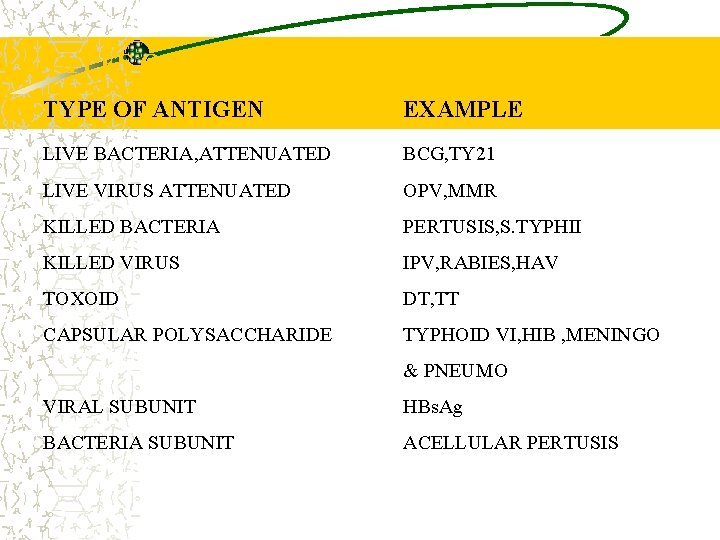
TYPES OF VACCINES TYPE OF ANTIGEN EXAMPLE LIVE BACTERIA, ATTENUATED BCG, TY 21 LIVE VIRUS ATTENUATED OPV, MMR KILLED BACTERIA PERTUSIS, S. TYPHII KILLED VIRUS IPV, RABIES, HAV TOXOID DT, TT CAPSULAR POLYSACCHARIDE TYPHOID VI, HIB , MENINGO & PNEUMO VIRAL SUBUNIT HBs. Ag BACTERIA SUBUNIT ACELLULAR PERTUSIS



Vaccines and equity Poorer / US community children at greater risk of illness and death from vaccine preventable diseases Routine vaccination promotes equity in child health

Tuberculosis (TB) is caused by the bacterium Mycobacterium tuberculosis which usually attacks the lungs, but can also affect other parts of the body, including the bones, joints, and brain TB is spread from one person to another through the air often when a person with the disease coughs or sneezes. TB spreads rapidly, especially in areas where people are living in crowded conditions,

Tuberculosis……. Immunization of infants with Bacille Calmette-Guérin vaccine (BCG) can protect against TB meningitis and other severe forms of TB in children less than five years old. BCG vaccine is not recommended after 24 months of age because the protection provided is variable and less certain.

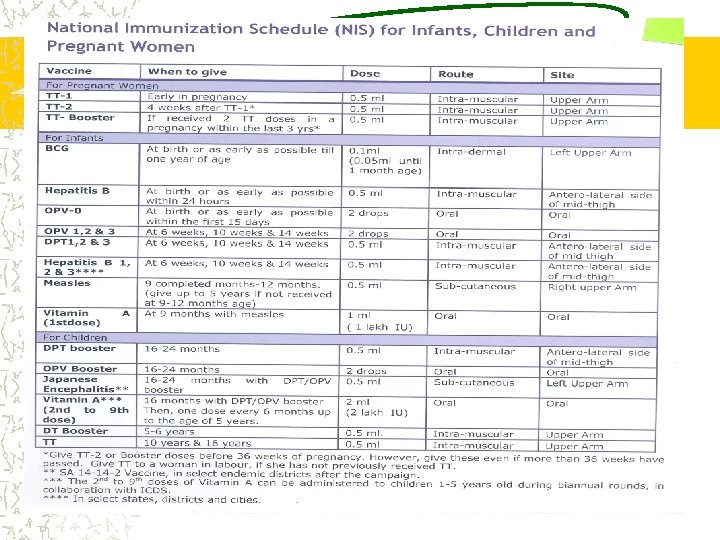
BCG injections ë Protect the child against childhood tuberculosis ë In institutional deliveries, immediately after birth ë In home deliveries, at one and half month along with the DPT injection and OPV

Poliomyelitis Disease of young children -80 -90% <5 yrs, majority below 2 years Clusters of susceptible needed to maintain circulation Infectivity typically one week before paralysis and 4 -6 weeks thereafter (peak first 2 weeks) Incubation period 7 -21 days (range 3 -35 days) Incubation period: Time from infection to appearance of symptoms Risk of paralysis increased by tonsillectomy, exercise, pregnancy, intramuscular injections

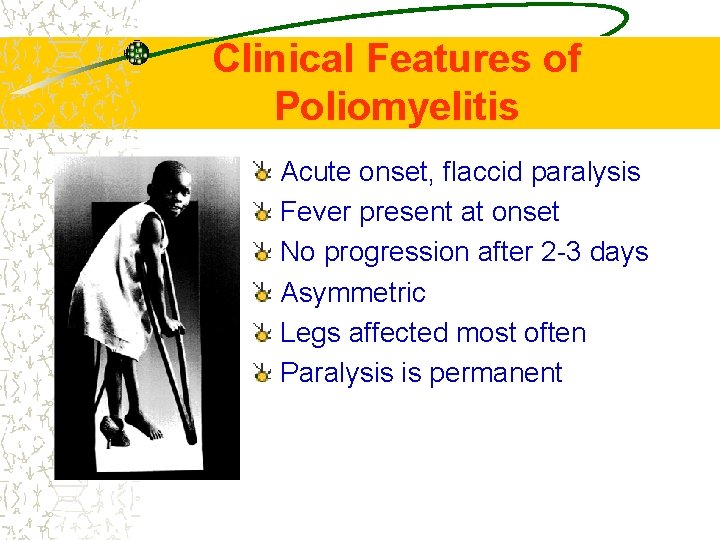
Clinical Features of Poliomyelitis Acute onset, flaccid paralysis Fever present at onset No progression after 2 -3 days Asymmetric Legs affected most often Paralysis is permanent

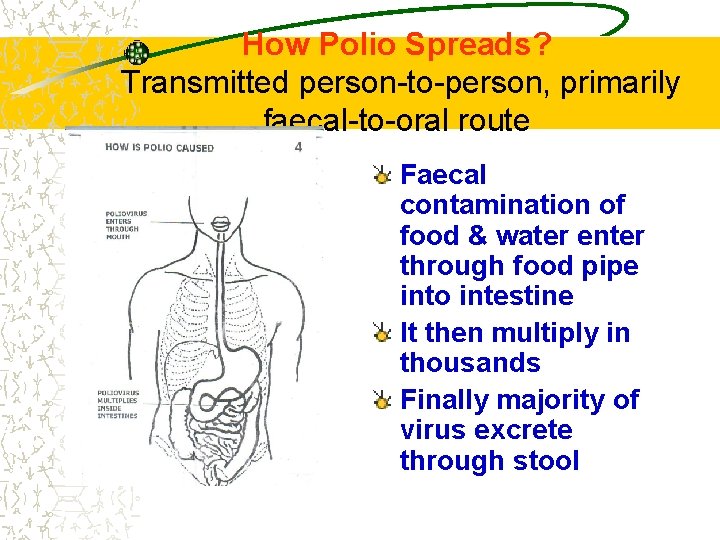
How Polio Spreads?

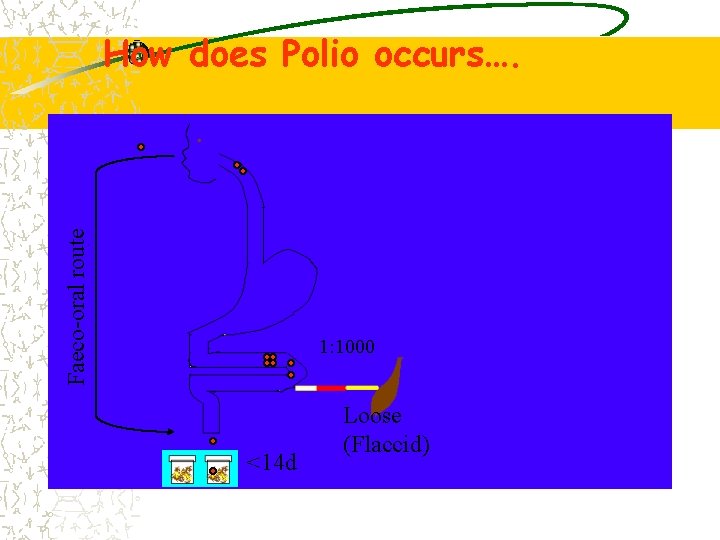
How Polio Spreads? Transmitted person-to-person, primarily faecal-to-oral route Faecal contamination of food & water enter through food pipe into intestine It then multiply in thousands Finally majority of virus excrete through stool

Faeco-oral route How does Polio occurs…. 1: 1000 <14 d Loose (Flaccid)

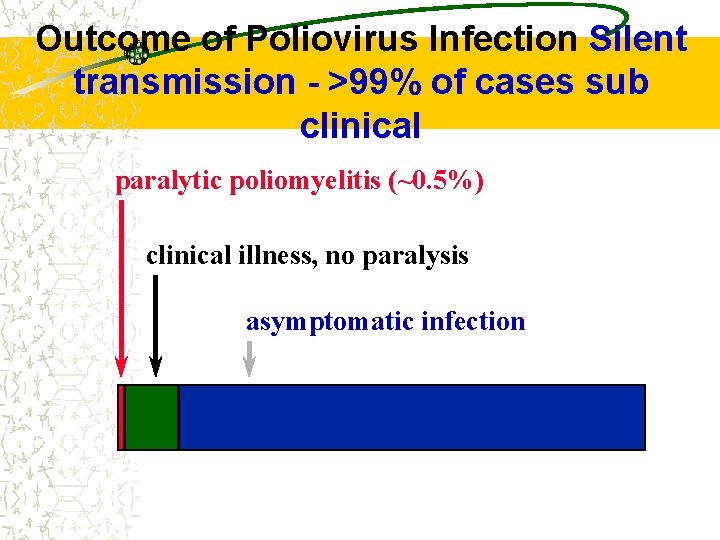
Outcome of Poliovirus Infection Silent transmission - >99% of cases sub clinical paralytic poliomyelitis (~0. 5%) clinical illness, no paralysis asymptomatic infection

MMR • MULTIDOSE , LIVE ATTENUATED VIRAL VACCINE • 15 MONTHS TO 5 years • DILUENT-DOUBLE DISTILLED WATER • MEASLES-1000 TCID-50 • MUMPS-5000 TCID-50 • RUBELLA 1000 TCID 50 • DOSE-0. 5 ML S/C

HEPATITIS B • RECOMBINANT • DOSE-0. 5 ML OR 10 MICROGRAM • ROUTE INTRAMUSCULAR • NOT TO BE GIVEN IN GLUTEAL REGION • BIRTH DOSE-INSTITUTIONAL DELIVERIES • 3 DOSES WITH DPT STARTING FROM 6 WEEKS TO 14 WEEKS

Tetanus Epidemiology and Prevention of Vaccine. Preventable Diseases National Immunization Program Centers for Disease Control and Prevention Revised March 2002

Clostridium tetani Spore-forming bacteria Spores found in soil, dust, animal feces; may persist for months to years

Tetanus Pathogenesis Toxin binds in central nervous system Leads to muscle contraction and spasm

Tetanus Clinical Features Incubation period 8 days (range, 3 -21 days) Generalized tetanus: descending symptoms of trismus (lockjaw), difficulty swallowing, muscle rigidity, spasms Spasms continue for 3 -4 weeks; complete recovery may take months

Neonatal Tetanus Generalized tetanus in newborn infant Infant born without protective passive immunity: Mother not immunized with Tetanus Toxoid immunization Almost all affected newborn die

Clinical Neonatal Tetanus

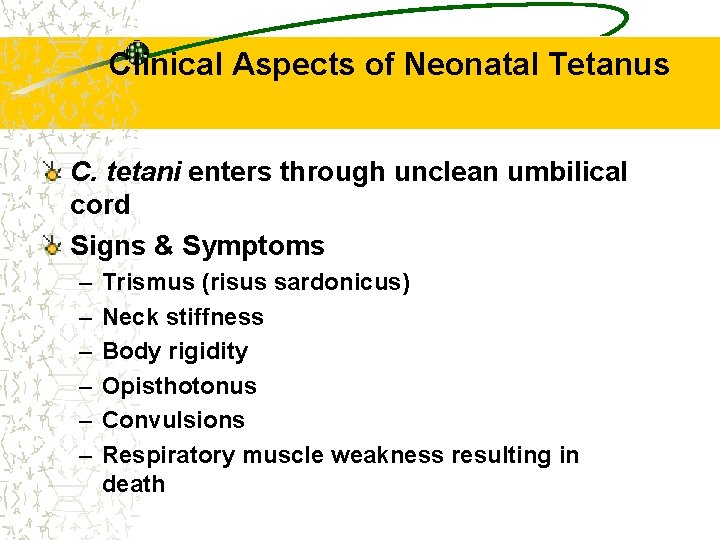
Clinical Aspects of Neonatal Tetanus C. tetani enters through unclean umbilical cord Signs & Symptoms – – – Trismus (risus sardonicus) Neck stiffness Body rigidity Opisthotonus Convulsions Respiratory muscle weakness resulting in death

Neonatal Tetanus Any newborn baby that - sucks and cries normally during the first 2 (two) days of life; - becomes ill between 3 and 28 days of life with BOTH 1. Inability to suck - and 2. Generalized muscle rigidity (stiffness)

How to Prevent Neonatal Tetanus Two complimentary strategies 1. Clean delivery - “ 5 cleans” • • • Clean delivery surface Clean hands Clean Thread Clean and New Blade Clean umbilical cord and stump care 2. Immunization of mother with TT

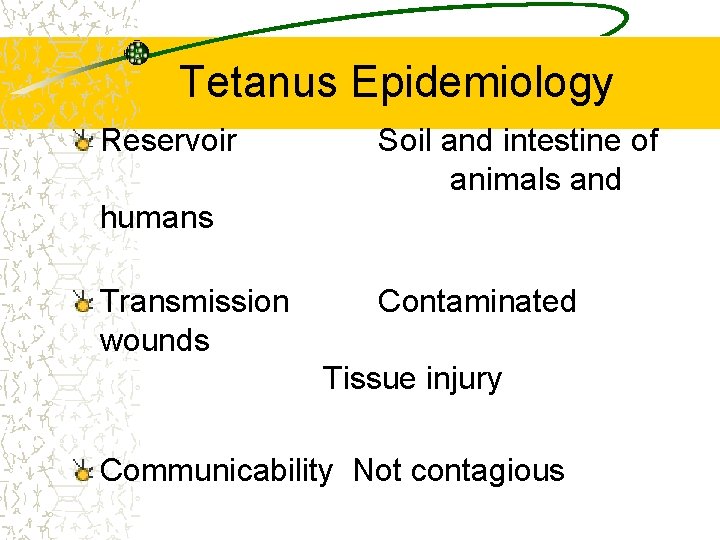
Tetanus Epidemiology Reservoir Soil and intestine of animals and humans Transmission wounds Contaminated Tissue injury Communicability Not contagious

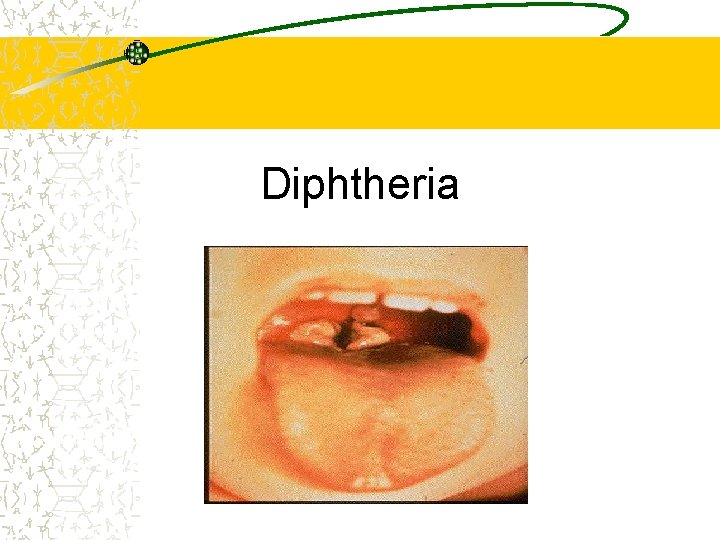
Diphtheria Epidemiology and Prevention of Vaccine. Preventable Diseases National Immunization Program Centers for Disease Control and Prevention Revised March 2002

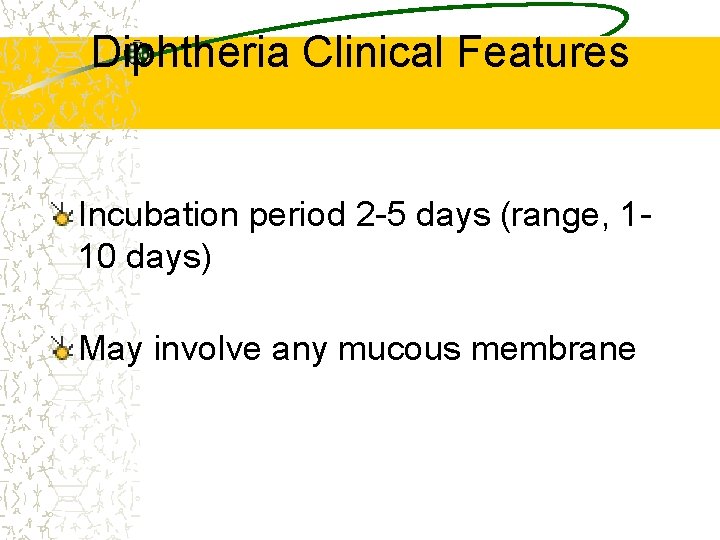
Diphtheria Clinical Features Incubation period 2 -5 days (range, 110 days) May involve any mucous membrane

Diphtheria Epidemiology Reservoir Human carriers Usually asymptomatic Transmission Respiratory Skin and fomites rarely Communicability Up to several weeks without antibiotics

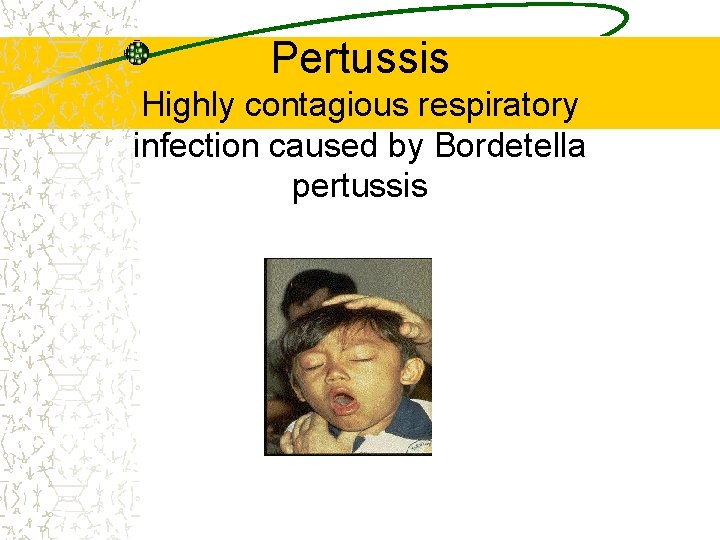
Pertussis Highly contagious respiratory infection caused by Bordetella pertussis Epidemiology and Prevention of Vaccine. Preventable Diseases National Immunization Program Centers for Disease Control and Prevention Revised August 2002

Pertussis Clinical Features Incubation period 5 -10 days (up to 21 days) Slow onset, similar to minor upper respiratory infection with nonspecific cough Fever usually minimal throughout course

Pertussis Clinical Features Primary stage 1 -2 weeks Paroxysmal cough stage 1 -6 weeks Convalescence months Weeks to

Pertussis Epidemiology Reservoir Human Adolescents and adults Transmission Respiratory droplets Airborne rare Communicability Maximum in catarrhal stage

DPT ë One dose gives very limited protection ë Two doses give 80 -% short term protection ë Three doses lead to sustainable protection

Measles Highly contagious viral illness caused by measles virus {Paramyxovirus (RNA)}

Measles Pathogenesis Respiratory transmission of virus Replication in nasopharynx and regional lymph nodes

Measles Clinical Features Incubation period 10 -12 days Fever, cough, coryza, conjunctivitis 2 -4 days after prodrome, 14 days after exposure Maculopapular rash Begins on face and head Persists 5 -6 days Complications: Diarrhea, Otitis media, Pneumonia, Encephalitis, Death

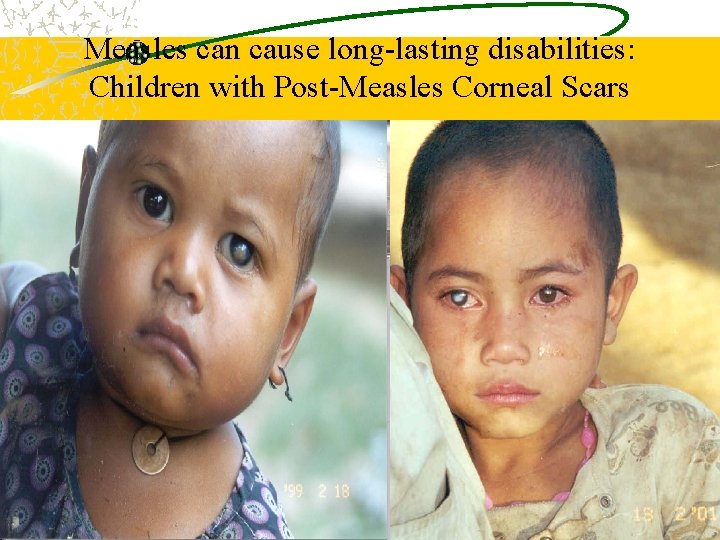
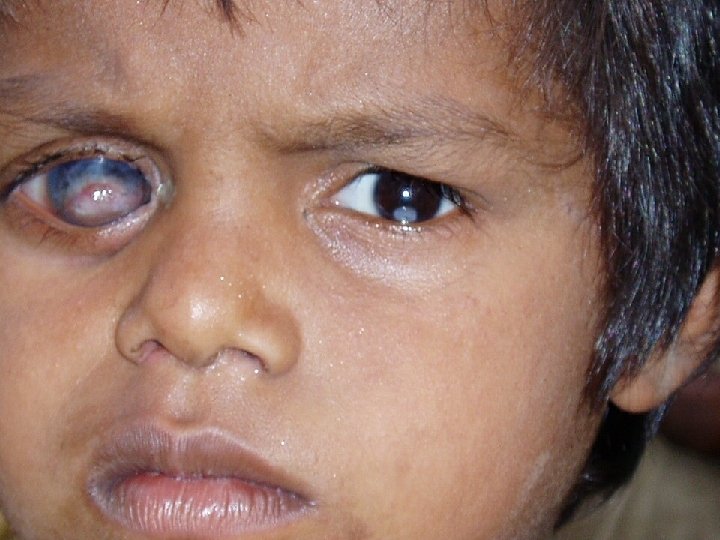
Measles can cause long-lasting disabilities: Children with Post-Measles Corneal Scars


Vitamin A Supplementation 9 to 12 months: First dose: With measles vaccine: 1 Lac IU ( 1 ml= ½ spoon) More than 12 to 59 months: Second to ninth dose at the intervals of six months : 2 Lac IU (2 ml= 1 spoon)

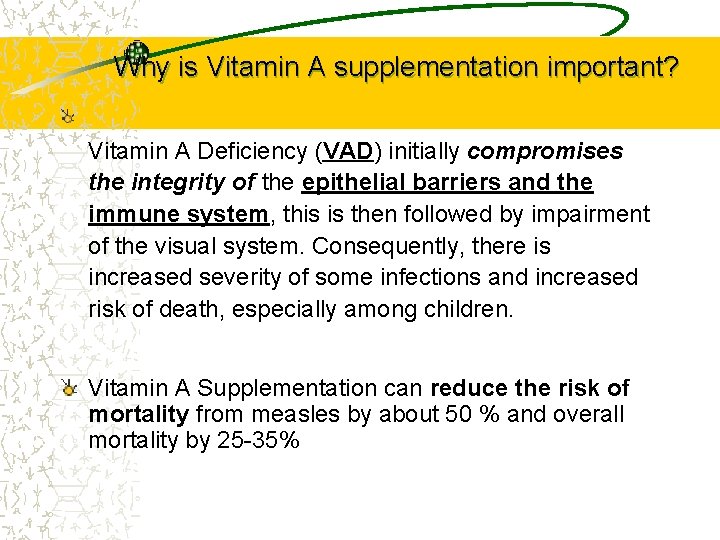
Why is Vitamin A supplementation important? Vitamin A Deficiency (VAD) initially compromises the integrity of the epithelial barriers and the immune system, this is then followed by impairment of the visual system. Consequently, there is increased severity of some infections and increased risk of death, especially among children. Vitamin A Supplementation can reduce the risk of mortality from measles by about 50 % and overall mortality by 25 -35%

Vitamin A Supplementation 9 to 12 months: First dose: With measles vaccine: 1 Lac IU ( 1 ml= ½ spoon) More than 12 to 60 months: Second to ninth dose at the intervals of six months : 2 Lac IU (2 ml= 1 spoon)

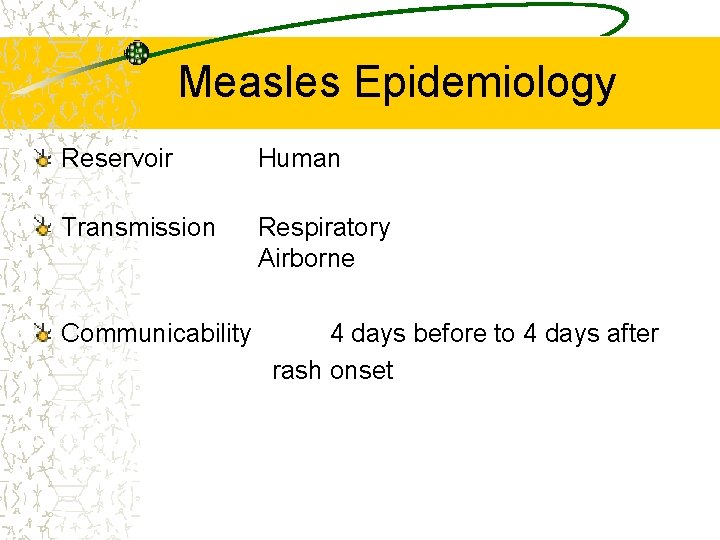
Measles Epidemiology Reservoir Human Transmission Respiratory Airborne Communicability 4 days before to 4 days after rash onset

Measles Vaccine Composition Live virus Duration of Immunity Lifelong Schedule One dose

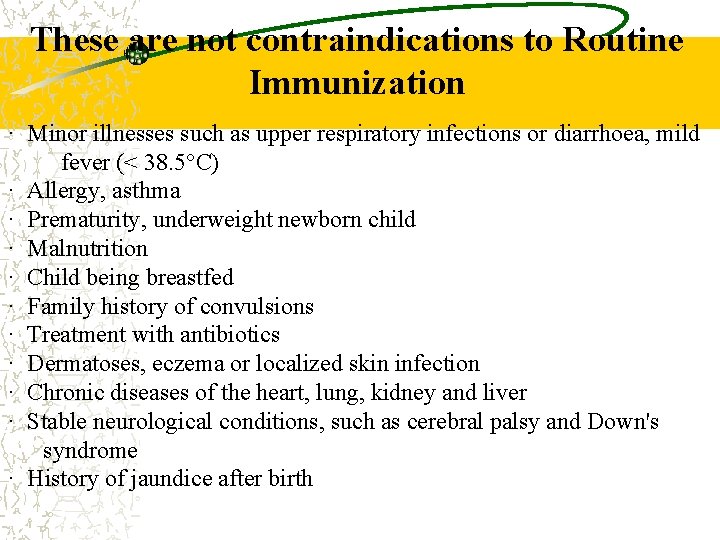
These are not contraindications to Routine Immunization · Minor illnesses such as upper respiratory infections or diarrhoea, mild fever (< 38. 5°C) · Allergy, asthma · Prematurity, underweight newborn child · Malnutrition · Child being breastfed · Family history of convulsions · Treatment with antibiotics · Dermatoses, eczema or localized skin infection · Chronic diseases of the heart, lung, kidney and liver · Stable neurological conditions, such as cerebral palsy and Down's syndrome · History of jaundice after birth

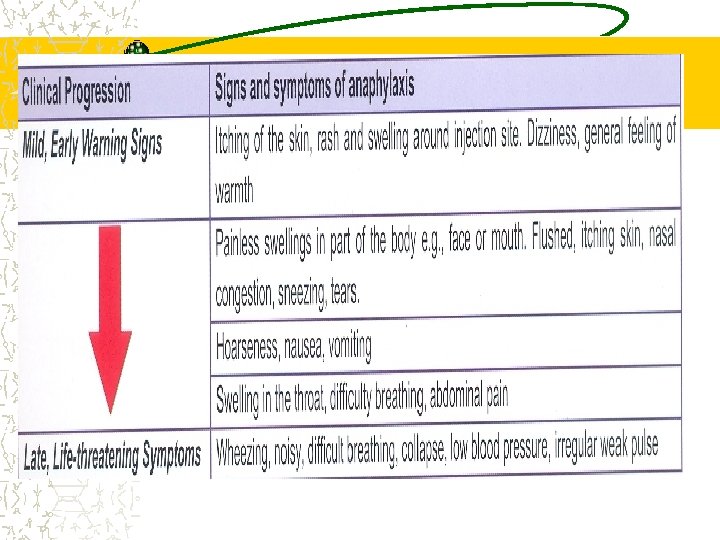
AEFI Vaccines are safe and effective Life threatening adverse events are extremely rare Mild side effects are commonly seen and can be self limiting and easily manageable Benefits of immunization greatly outweighs the risks of AEFI Majority are due to unsafe injection practices and procedures




AEFI---- Rare, more severe reactions Include : § seizures, thrombocytopenia, § hypotonic-hypo responsive episodes, § persistent inconsolable screaming In most cases they are self-limiting and lead to no long-term problems Anaphylaxis, while potentially fatal, is

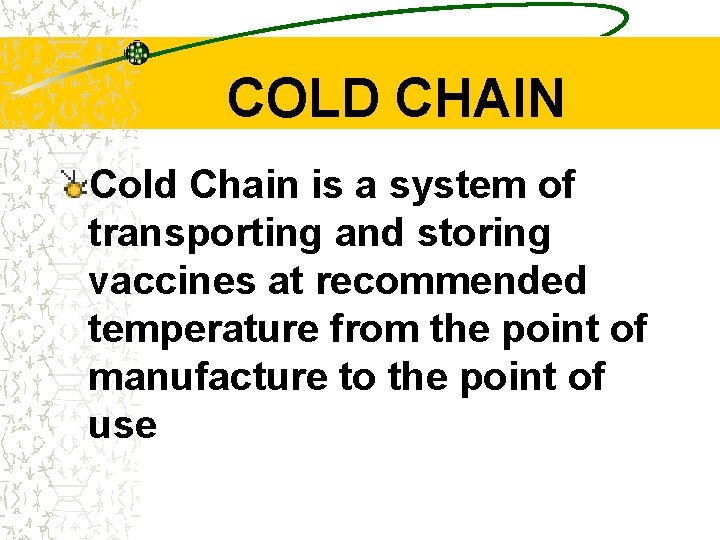
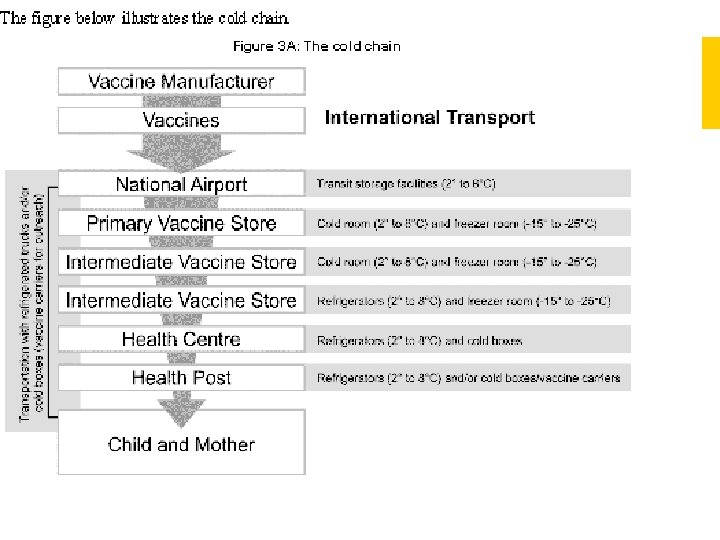
COLD CHAIN Cold Chain is a system of transporting and storing vaccines at recommended temperature from the point of manufacture to the point of use

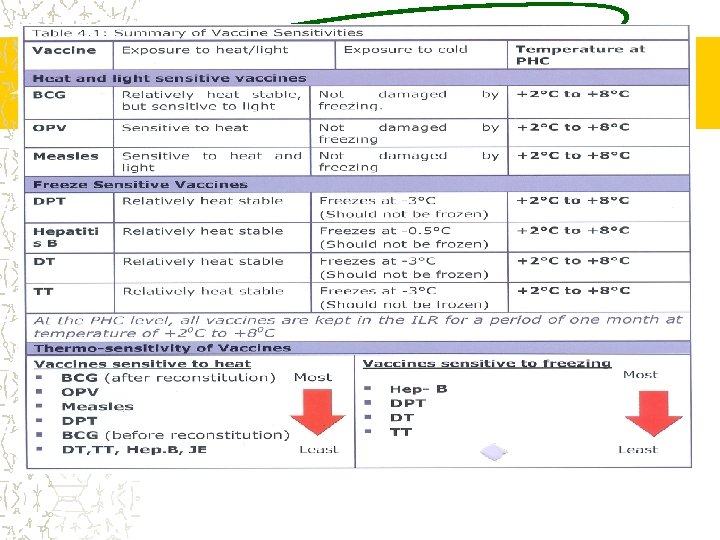
COLD CHAIN All Vaccines tend to lose potency on exposure to heat above +80 C Some Vaccines lose potency when exposed to freezing temperatures The damage is irreversible



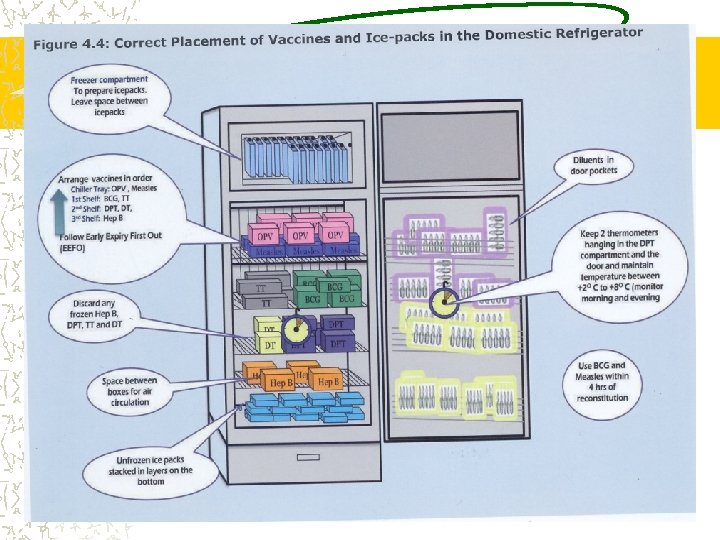
COLD CHAIN EQUIPMENTS WALK IN COOLERS & FREEZERS ICE LINED REFRIGERATORS DEEP FREEZERS VACCINE CARRIERS DAY CARRIERS COLD BOXES DOMESTIC REFRIGERATORS-IN DELHI


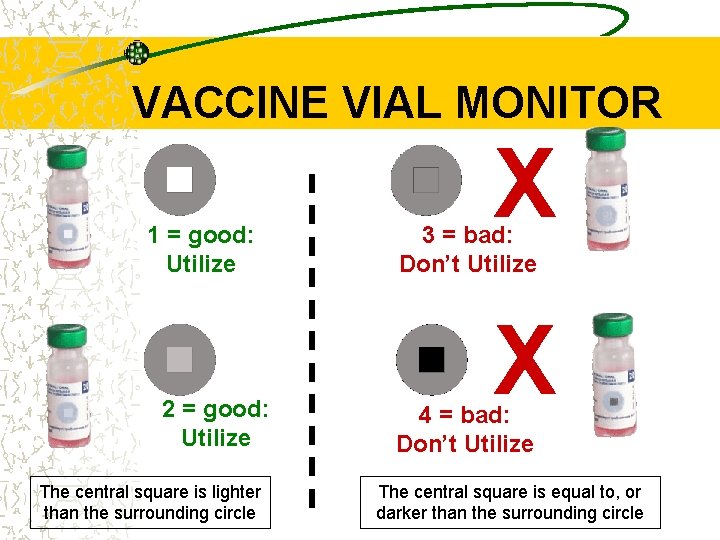
VACCINE VIAL MONITOR 1 = good: Utilize 2 = good: Utilize The central square is lighter than the surrounding circle X 3 = bad: Don’t Utilize X 4 = bad: Don’t Utilize The central square is equal to, or darker than the surrounding circle

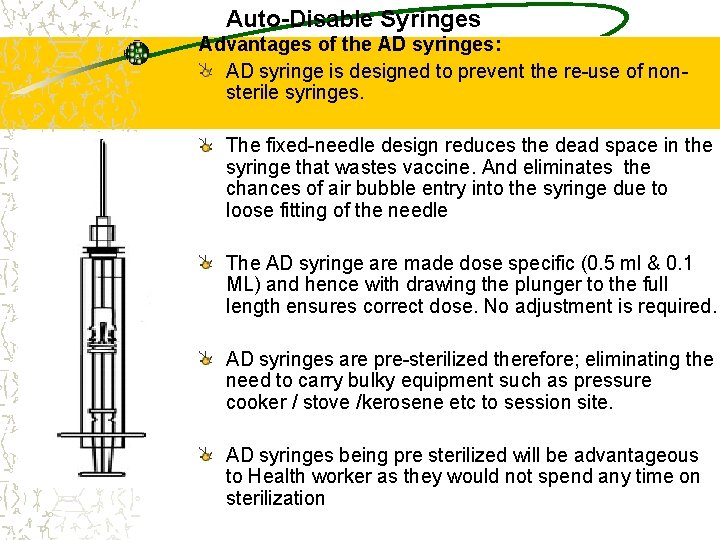
AD SYRINGES • Impossible to reuse • Lowest risk of person to person transmission of blood borne infections. • Fixed needle reduces dead space so less wastages. Also eliminates chances of air bubble entry due to loose fitting earlier. • Dose specific-ensure correct dose • Presterilized-no use of bulky equipment.

AD SYRINGE Do not use if damaged/torn Tear pack from plunger side. Remove needle cover& discard Take dose – do not touch needle or rubber cap Draw dose. If bubble just shake, administer. Push plunger completely. Do not recap.

AD SYRINGE Cut the needle immediately from the hub with the help of the hub cutter. It will go to the white sturdy container. NO UNTREATED BIO-MEDICAL WASTE SHALL BE STORED BEYOND A PRRIOD OF 48 HOURS.

Auto-Disable Syringes Advantages of the AD syringes: AD syringe is designed to prevent the re-use of nonsterile syringes. The fixed-needle design reduces the dead space in the syringe that wastes vaccine. And eliminates the chances of air bubble entry into the syringe due to loose fitting of the needle The AD syringe are made dose specific (0. 5 ml & 0. 1 ML) and hence with drawing the plunger to the full length ensures correct dose. No adjustment is required. AD syringes are pre-sterilized therefore; eliminating the need to carry bulky equipment such as pressure cooker / stove /kerosene etc to session site. AD syringes being pre sterilized will be advantageous to Health worker as they would not spend any time on sterilization

WASTE GENERATION AD SYRINGES TO BE USED WHERE SAFE DISPOSAL POSSIBLE WASTE CONSISTS OF: 1. PACKAGING MATERIAL 2. SYRINGES 3. NEEDLES 4. BROKEN/DISCARDED VIALS

What is Surveillance WHO – “The continuous scrutiny of the factors that determine the occurrence of disease and other conditions of ill health. Surveillance is essential for effective control and prevention and includes the collection, analysis, interpretation and distribution of relevant data for action” “Data collection for Action"

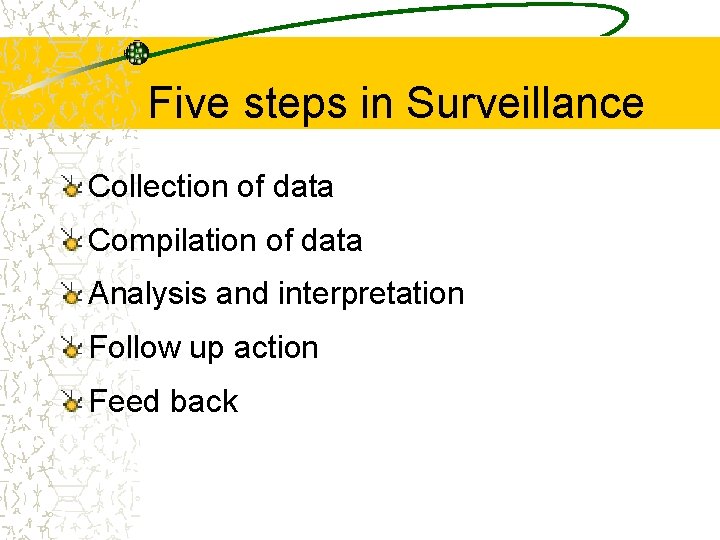
Five steps in Surveillance Collection of data Compilation of data Analysis and interpretation Follow up action Feed back

Prerequisites for effective Surveillance Use of Standard Case Definitions Ensuring Regularity of the Reports Action on Reports Medical Officer must be clear about: – – – What information to gather How often to compile & analyze the data How often & to whom to report What Performa or formats to use What action to take
