Round table Regulation of Medical Devices in the

Round table Regulation of Medical Devices in the Americas: challenges and opportunities VII PANDRH Conference

Working group on Medical Devices • Group created in 2012 to work on the strengthening of the regulatory capacity of medical devices, with representatives from Argentina, Brazil, Canada, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Honduras, Mexico, Panama, Peru and Uruguay • First meeting in Habana, July 2012, to establish the priorities: mapping the current situation of medical devices regulation in the Region and establish a baseline; develop a way to effectively exchange information amongst agencies; capacity building; develop a assessment tool for the evaluation of regulatory authorities; and interaction HTA-Regulation

First results • Presented at the 2 nd meeting in Buenos Aires (June 2013): - mapping: a template with 45 questions was developed and applied to 14 countries - exchange of information amongst agencies: creation of a community of practice within the PRAIS was created - capacity building: participation in the International Regulatory Forum and proposal for a virtual course on Regulation and HTA - interaction Regulation-HTA: pilot project PAHO/USAID
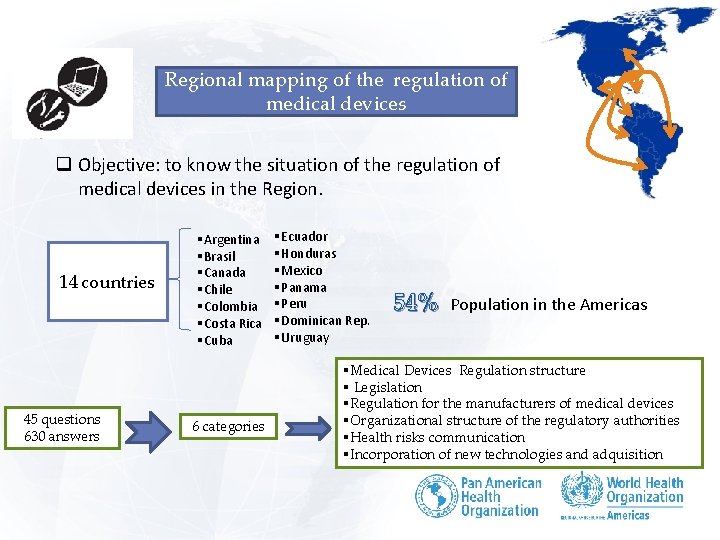
Regional mapping of the regulation of medical devices q Objective: to know the situation of the regulation of medical devices in the Region. 14 countries 45 questions 630 answers § Argentina § Brasil § Canada § Chile § Colombia § Costa Rica § Cuba 6 categories § Ecuador § Honduras § Mexico § Panama § Peru § Dominican Rep. § Uruguay 54% Population in the Americas § Medical Devices Regulation structure § Legislation § Regulation for the manufacturers of medical devices § Organizational structure of the regulatory authorities § Health risks communication § Incorporation of new technologies and adquisition
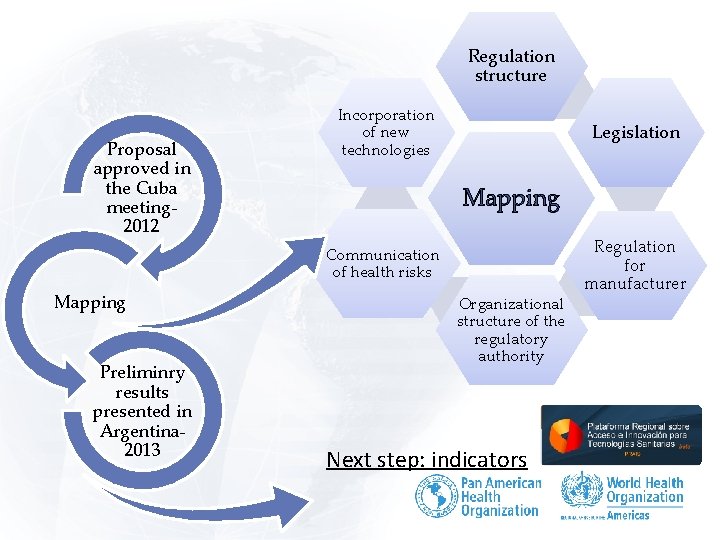
Regulation structure Proposal approved in the Cuba meeting 2012 Incorporation of new technologies Legislation Mapping Communication of health risks Mapping Preliminry results presented in Argentina 2013 Organizational structure of the regulatory authority Next step: indicators Regulation for manufacturer

93% Some Results Have some kind of medical devices regulation 85% Have registration of the manufacturers 43% Continuous capacity building programs for those working on medical devices 24% Specific regulation for medical equipments 93% Have post marketing surveillance programs 64% Marketing authorization for 100% of the devices 50% Quality controls only after receiving reports 64% Have stretegic alliances with other institutions for the strengthening of the regulatory capacity
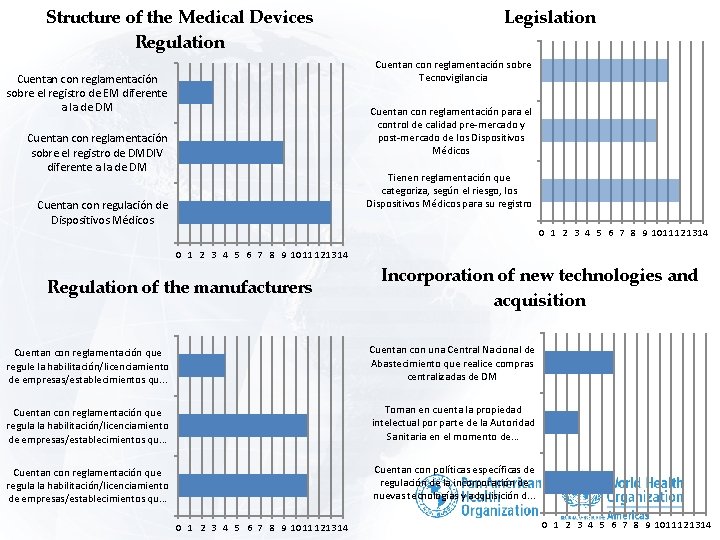
Structure of the Medical Devices Regulation Legislation Cuentan con reglamentación sobre Tecnovigilancia Cuentan con reglamentación sobre el registro de EM diferente a la de DM Cuentan con reglamentación para el control de calidad pre-mercado y post-mercado de los Dispositivos Médicos Cuentan con reglamentación sobre el registro de DMDIV diferente a la de DM Tienen reglamentación que categoriza, según el riesgo, los Dispositivos Médicos para su registro Cuentan con regulación de Dispositivos Médicos 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Regulation of the manufacturers Incorporation of new technologies and acquisition Cuentan con reglamentación que regule la habilitación/licenciamiento de empresas/establecimientos qu. . . Cuentan con una Central Nacional de Abastecimiento que realice compras centralizadas de DM Cuentan con reglamentación que regula la habilitación/licenciamiento de empresas/establecimientos qu. . . Toman en cuenta la propiedad intelectual por parte de la Autoridad Sanitaria en el momento de. . . Cuentan con reglamentación que regula la habilitación/licenciamiento de empresas/establecimientos qu. . . Cuentan con políticas específicas de regulación de la incorporación de nuevas tecnologías y adquisición d. . . 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
- Slides: 7