ROTATIONAL SPECTROSCOPY Microwave interactions Quantum energy of microwave


- Slides: 25

ROTATIONAL SPECTROSCOPY

Microwave interactions • Quantum energy of microwave photons (0. 00001 -0. 001 e. V) matches the ranges of energies separating quantum states of molecular rotations and torsion • Note that rotational motion of molecules is quantized, like electronic and vibrational transitions associated absorption/emission lines • Absorption of microwave radiation causes heating due to increased molecular rotational activity

PURE ROTATIONAL SPECTRA OF DIATOMIC MOLECULES Basic concepts Rotational energies of molecules are quantized (i. e. only have definite energies) E = h E, energy in J; h Planck’s constant, Js; rotational frequency, Hz. The range of rotational frequencies is about 8 x 1010 - 4 x 1011 Hz, which corresponds to wavelengths, ~ 0. 75 - 3. 75 mm. These wavelengths fall in the microwave region of the electromagnetic spectrum.

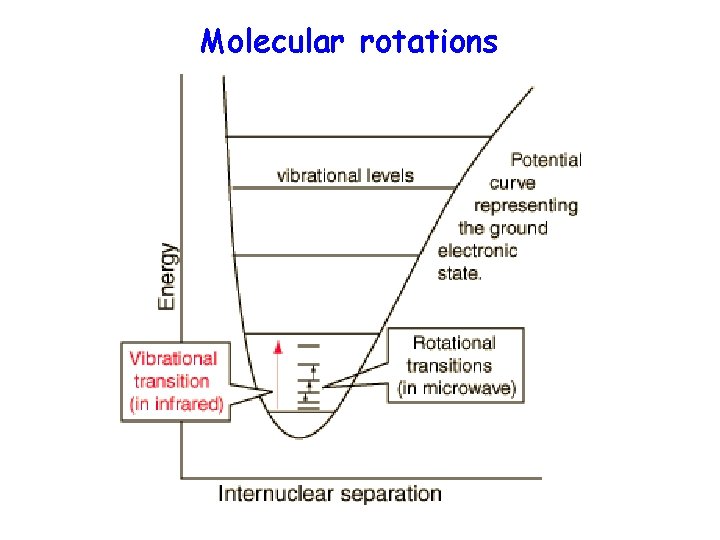
Molecular rotations

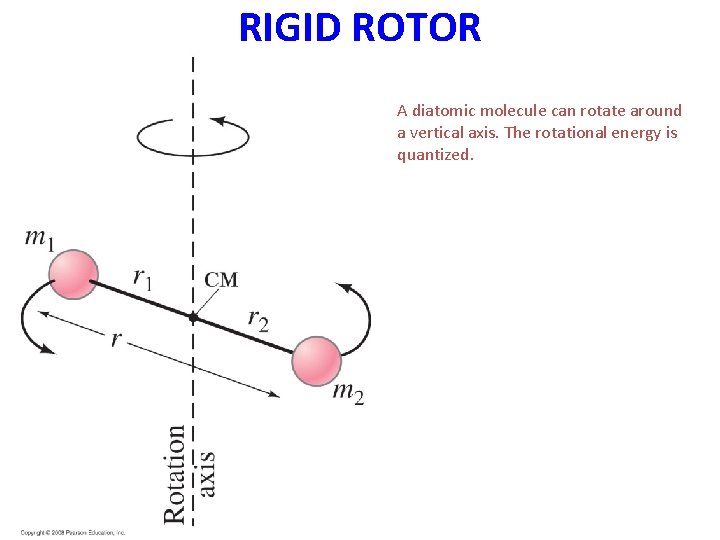
RIGID ROTOR Figure 40 -16 goes here. A diatomic molecule can rotate around a vertical axis. The rotational energy is quantized.

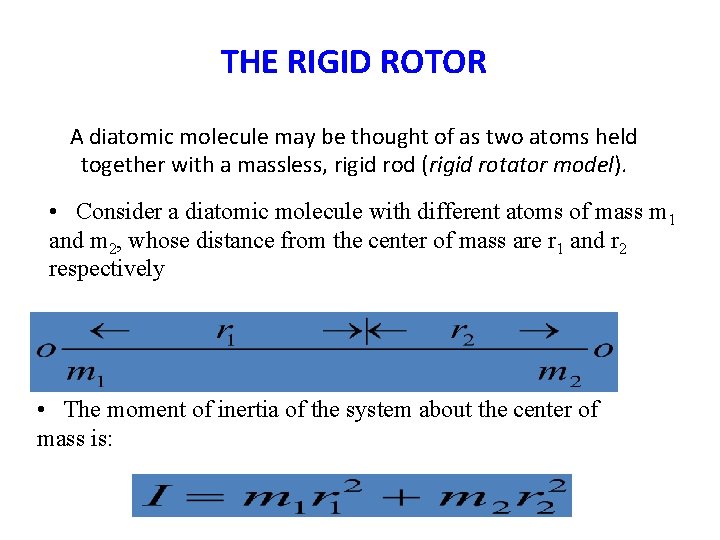
THE RIGID ROTOR A diatomic molecule may be thought of as two atoms held together with a massless, rigid rod (rigid rotator model). • Consider a diatomic molecule with different atoms of mass m 1 and m 2, whose distance from the center of mass are r 1 and r 2 respectively • The moment of inertia of the system about the center of mass is:

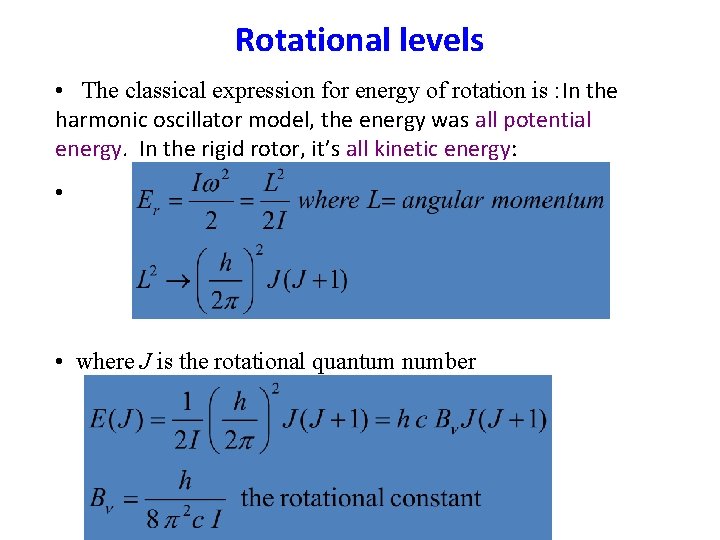
Rotational levels • The classical expression for energy of rotation is : In the harmonic oscillator model, the energy was all potential energy. In the rigid rotor, it’s all kinetic energy: • • where J is the rotational quantum number

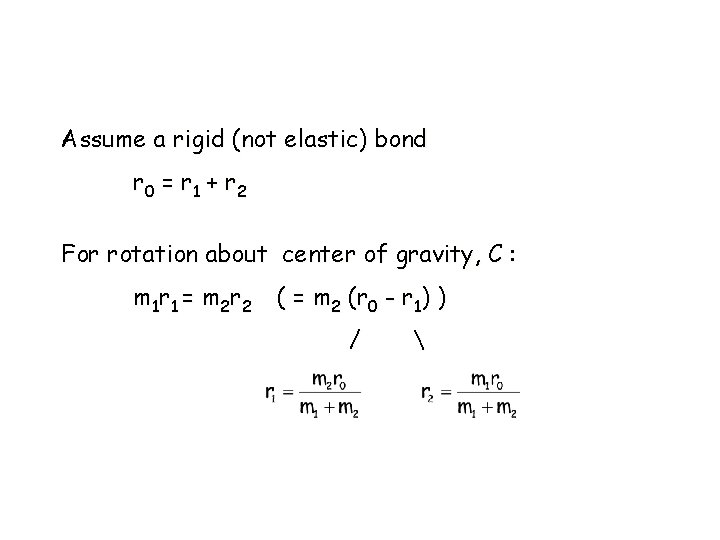
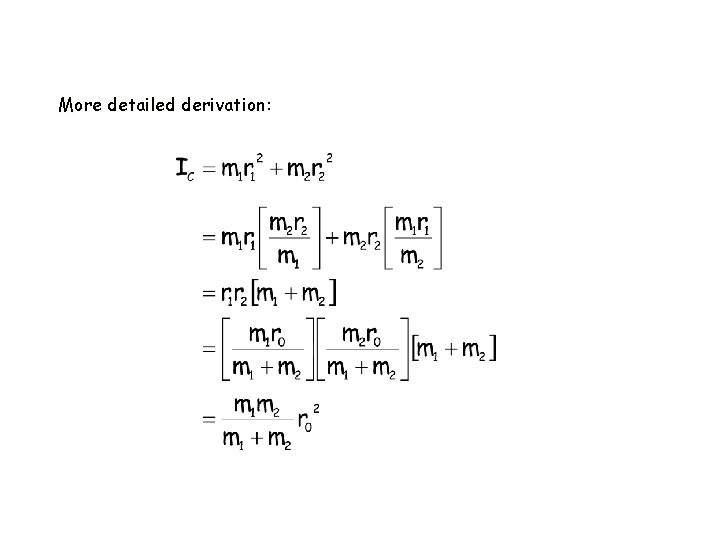
Assume a rigid (not elastic) bond r 0 = r 1 + r 2 For rotation about center of gravity, C : m 1 r 1 = m 2 r 2 ( = m 2 (r 0 - r 1) ) /

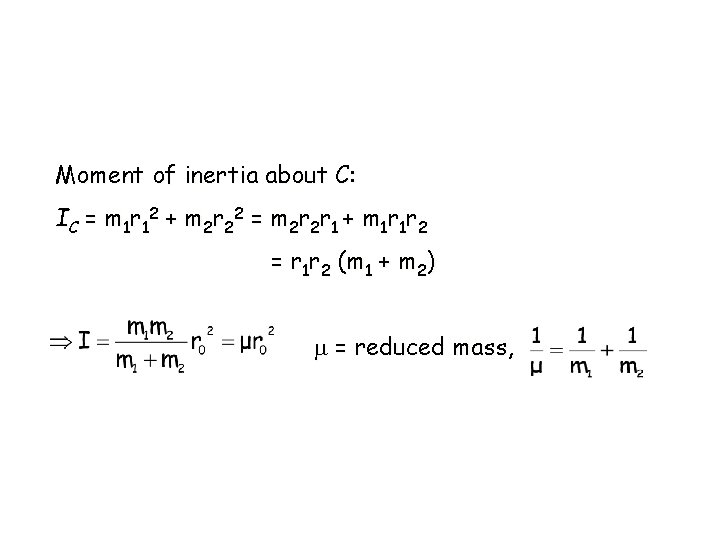
Moment of inertia about C: I C = m 1 r 12 + m 2 r 2 2 = m 2 r 1 + m 1 r 1 r 2 = r 1 r 2 (m 1 + m 2) = reduced mass,

More detailed derivation:

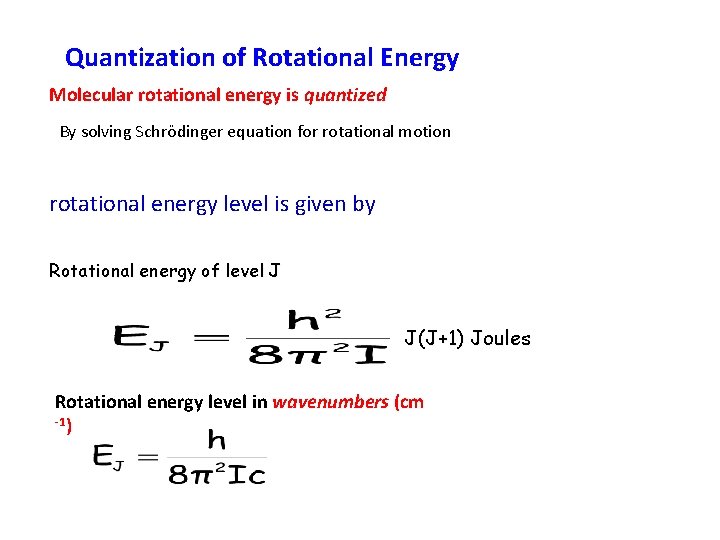
Quantization of Rotational Energy Molecular rotational energy is quantized By solving Schrödinger equation for rotational motion rotational energy level is given by Rotational energy of level J J(J+1) Joules Rotational energy level in wavenumbers (cm -1)

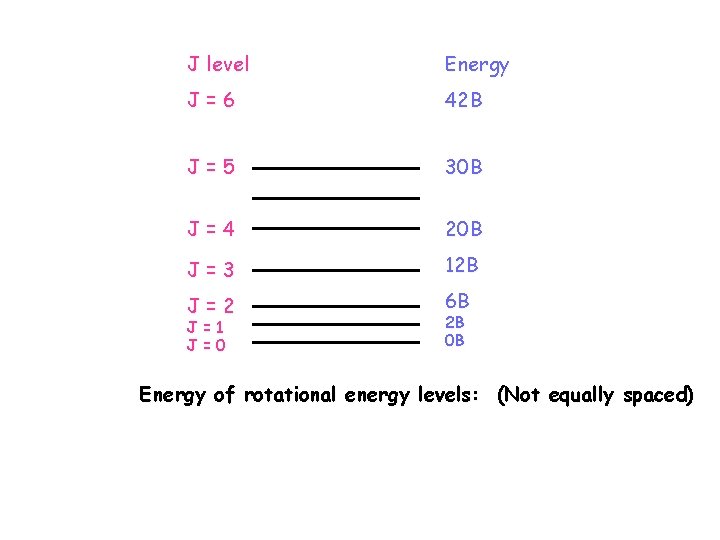
Where J is the rotational quantum number, having the values 0, 1 , 2…. Note that J = 0 is the lowest level, and the molecule is not rotating in this level. Now the rotational frequency is the same as the frequency of the microwave radiation need to cause the rotation: / Hz = E/h or in energy units: So , where c is in cms-1. J(J+1) cm-1 = BJ(J+1) cm-1 B is called the rotational constant for a given molecule. Its units are cm-1, since J is just a quantum number (label).

J level Energy J=6 42 B J=5 30 B J=4 20 B J=3 12 B J=2 6 B J=1 J=0 2 B 0 B Energy of rotational energy levels: (Not equally spaced)

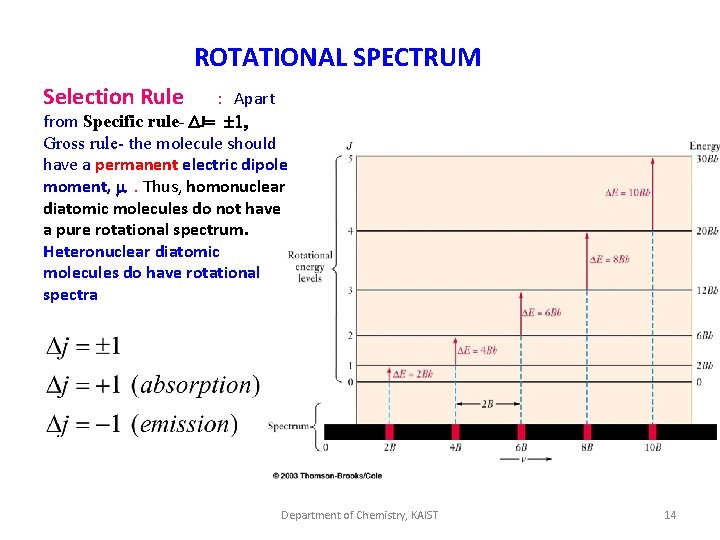
ROTATIONAL SPECTRUM Selection Rule : Apart from Specific rule- DJ= 1, Gross rule- the molecule should have a permanent electric dipole moment, m. Thus, homonuclear diatomic molecules do not have a pure rotational spectrum. Heteronuclear diatomic molecules do have rotational spectra Department of Chemistry, KAIST 14

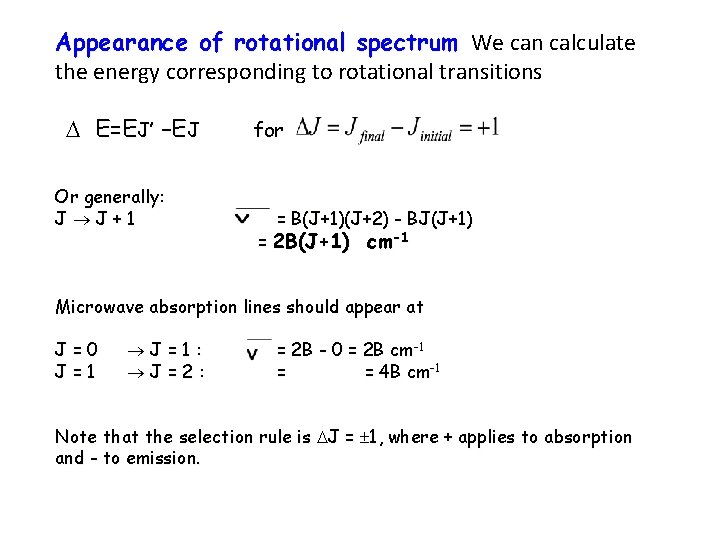
Appearance of rotational spectrum We can calculate the energy corresponding to rotational transitions E=EJ’ –EJ Or generally: J J+1 for = B(J+1)(J+2) - BJ(J+1) = 2 B(J+1) cm-1 Microwave absorption lines should appear at J=0 J=1: J=2: = 2 B - 0 = 2 B cm-1 = = 4 B cm-1 Note that the selection rule is J = 1, where + applies to absorption and - to emission.

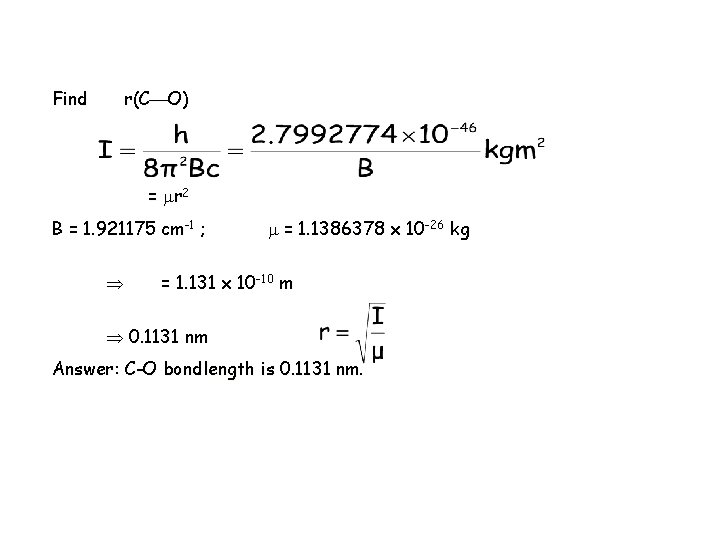
Find r(C O) = r 2 B = 1. 921175 cm-1 ; = 1. 1386378 x 10 -26 kg = 1. 131 x 10 -10 m 0. 1131 nm Answer: C-O bondlength is 0. 1131 nm.

Relative Intensities of rotation spectral lines Now we understand the locations (positions) of lines in the microwave spectrum, we can see which lines are strongest. J BJ(J+1) J=0 0 Intensity depends upon two factors:

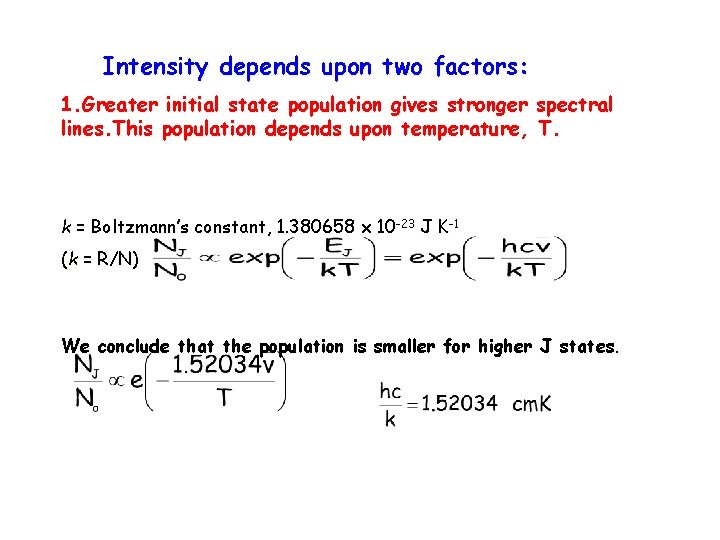
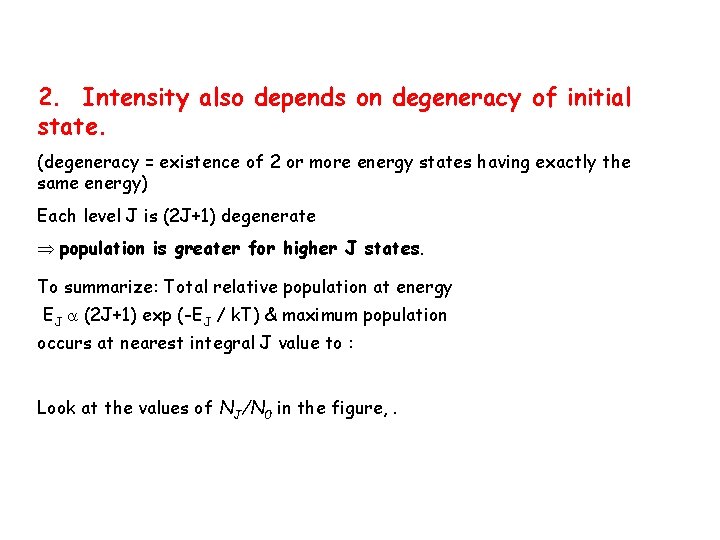
Intensity depends upon two factors: 1. Greater initial state population gives stronger lines. This population depends upon temperature, spectral T. k = Boltzmann’s constant, 1. 380658 x 10 -23 J K-1 (k = R/N) We conclude that the population is smaller for higher J states.

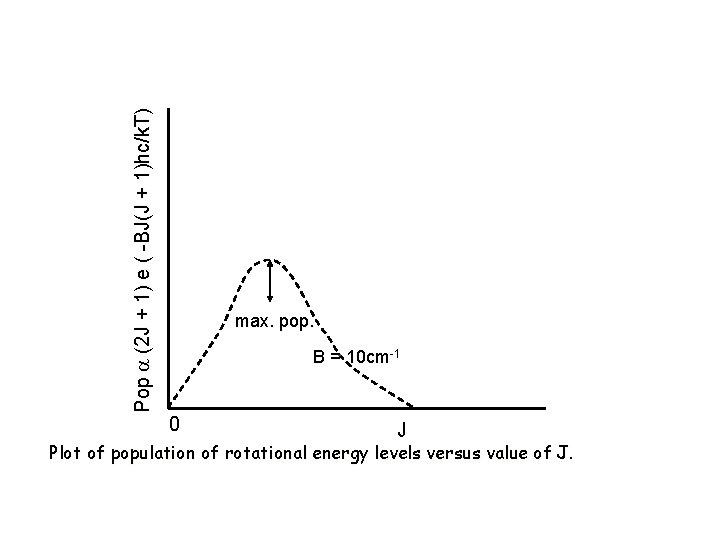
2. Intensity also depends on degeneracy of initial state. (degeneracy = existence of 2 or more energy states having exactly the same energy) Each level J is (2 J+1) degenerate population is greater for higher J states. To summarize: Total relative population at energy EJ (2 J+1) exp (-EJ / k. T) & maximum population occurs at nearest integral J value to : Look at the values of NJ/N 0 in the figure, .

Pop (2 J + 1) e ( -BJ(J + 1)hc/k. T) max. pop. B = 10 cm-1 0 J Plot of population of rotational energy levels versus value of J.

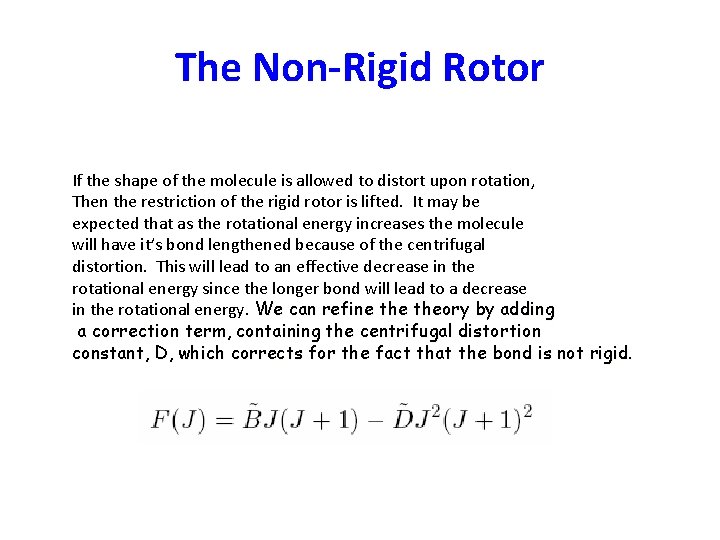
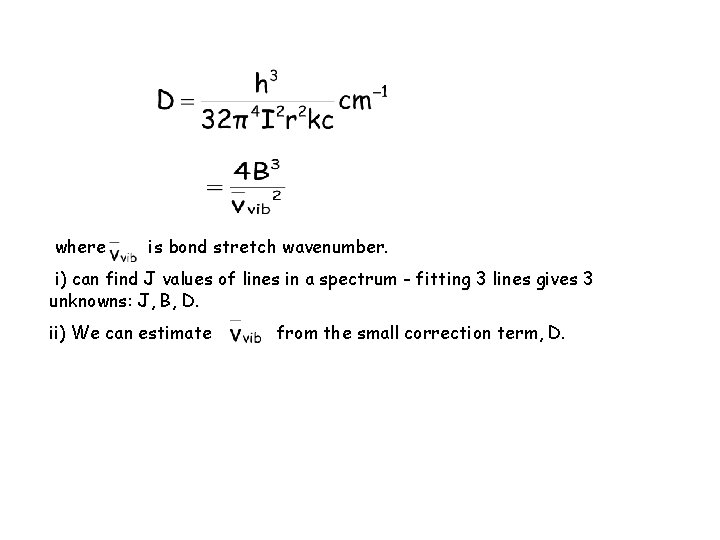
The Non-Rigid Rotor If the shape of the molecule is allowed to distort upon rotation, Then the restriction of the rigid rotor is lifted. It may be expected that as the rotational energy increases the molecule will have it’s bond lengthened because of the centrifugal distortion. This will lead to an effective decrease in the rotational energy since the longer bond will lead to a decrease in the rotational energy. We can refine theory by adding a correction term, containing the centrifugal distortion constant, D, which corrects for the fact that the bond is not rigid.

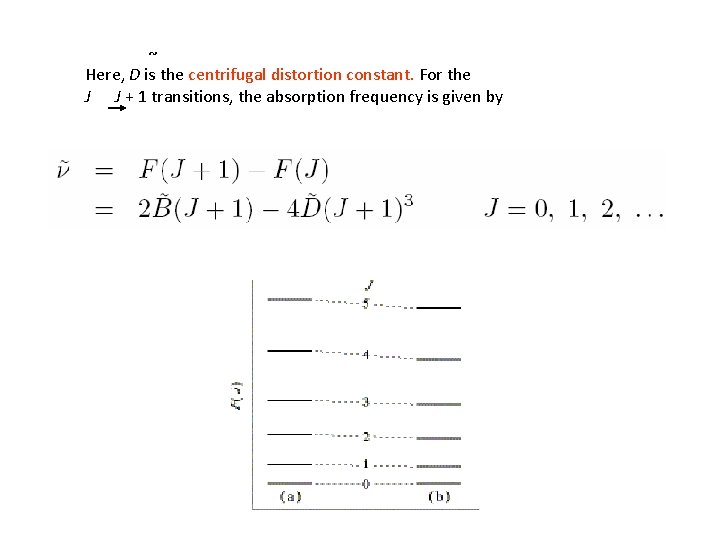
~ Here, D is the centrifugal distortion constant. For the J J + 1 transitions, the absorption frequency is given by

where is bond stretch wavenumber. i) can find J values of lines in a spectrum - fitting 3 lines gives 3 unknowns: J, B, D. ii) We can estimate from the small correction term, D.

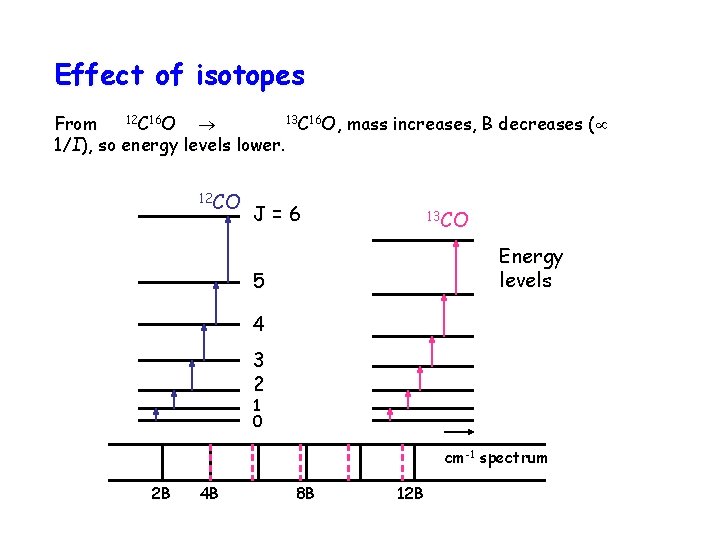
Effect of isotopes 12 C 16 O 13 C 16 O, mass increases, B decreases ( From 1/I), so energy levels lower. 12 CO J=6 13 CO Energy levels 5 4 3 2 1 0 cm-1 spectrum 2 B 4 B 8 B 12 B

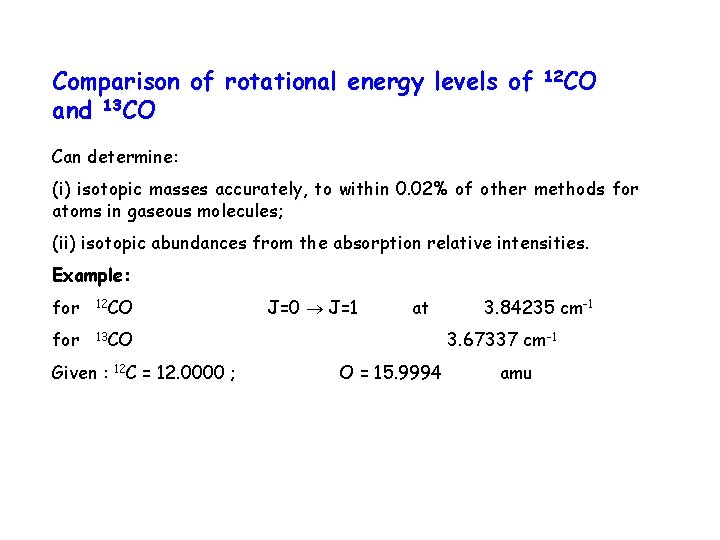
Comparison of rotational energy levels of and 13 CO 12 CO Can determine: (i) isotopic masses accurately, to within 0. 02% of other methods for atoms in gaseous molecules; (ii) isotopic abundances from the absorption relative intensities. Example: for 12 CO for 13 CO Given : 12 C = 12. 0000 ; J=0 J=1 at 3. 84235 cm-1 3. 67337 cm-1 O = 15. 9994 amu