Rotational heat capacity of Hydrogen molecule Moment of








![= d/d. T[Lk. T 2 dlnf/d. T] Since lnf = constant Cv(ortho) = 0 = d/d. T[Lk. T 2 dlnf/d. T] Since lnf = constant Cv(ortho) = 0](https://slidetodoc.com/presentation_image_h2/92bf35ab564e58e8759073ca3ca843e4/image-23.jpg)
- Slides: 23

Rotational heat capacity of Hydrogen molecule

• Moment of inertia of hydrogen very small so rotational quanta too large • so classical treatment not used • rotational partition function calculated only by summation • But calculated value does not agree with experimental value

• Difference is due to two nuclear spin isomers for hydrogen molecule • Nuclear spin of the two protons can be paired to get either symmetric combination (spin parallel) or antisymmetric combination (spin opposed) • Former is ortho and latter para hydrogen

• Wave number ν= h. J/4π2 Ic for rotational space of diatomic molecule • Spectra of ortho hydrogen has fairly intense lines corresponding to odd values of J • Para hydrogen has less intense lines corresponding to even value of J • Spectra of hydrogen has alternating intense and less intense rotational lines – shows that mixture contains ortho(75%) and para hydrogen

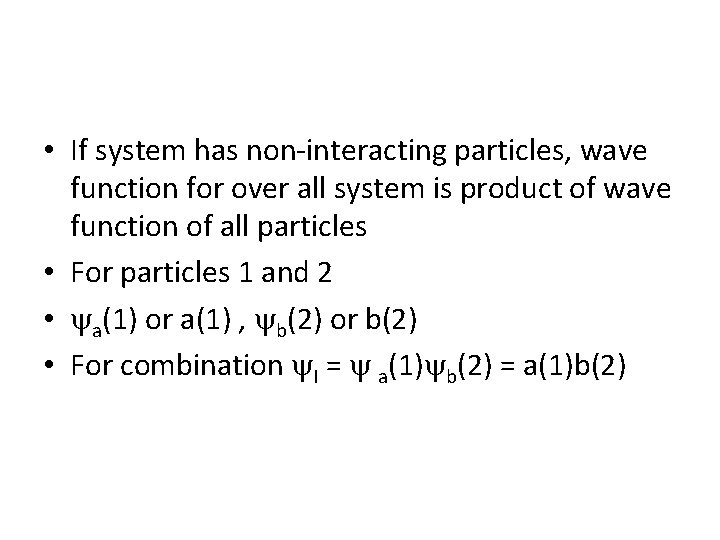
• If system has non-interacting particles, wave function for over all system is product of wave function of all particles • For particles 1 and 2 • a(1) or a(1) , b(2) or b(2) • For combination I = a(1) b(2) = a(1)b(2)

• If indistingushiable particles, same wave function can be available for both • So II = a(2) b(1) = a(2)b(1) is also valid • But by quantum mechanics this is not acceptable

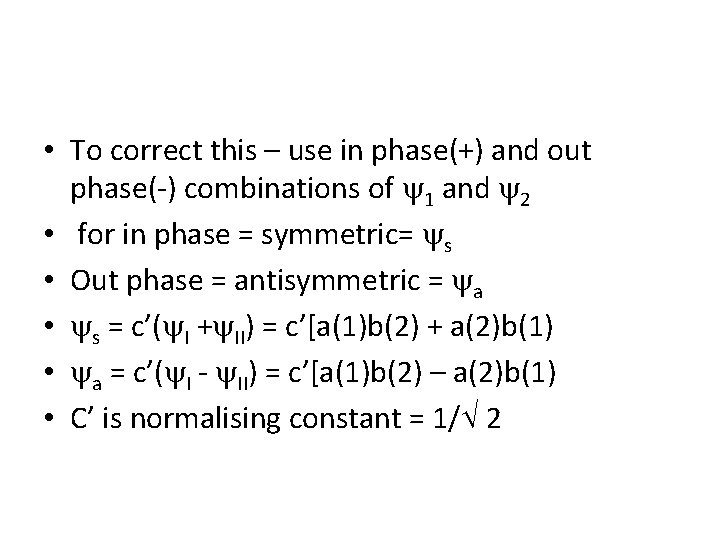
• To correct this – use in phase(+) and out phase(-) combinations of 1 and 2 • for in phase = symmetric= s • Out phase = antisymmetric = a • s = c’( I + II) = c’[a(1)b(2) + a(2)b(1) • a = c’( I - II) = c’[a(1)b(2) – a(2)b(1) • C’ is normalising constant = 1/ 2

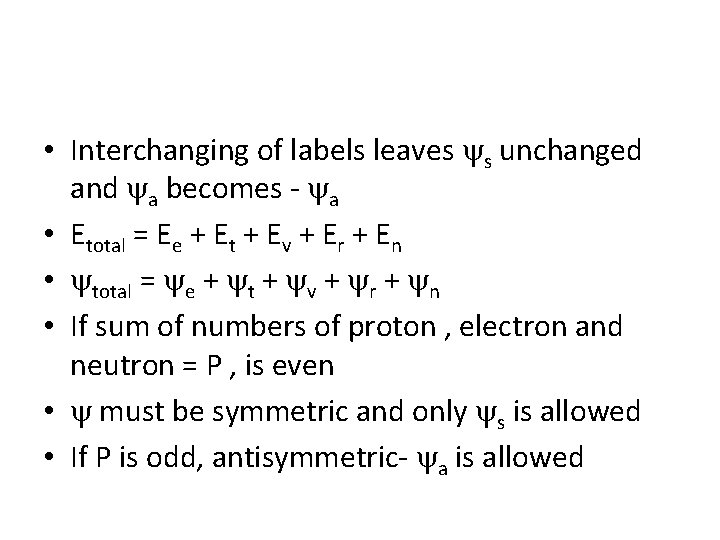
• Interchanging of labels leaves s unchanged and a becomes - a • Etotal = Ee + Et + Ev + Er + En • total = e + t + v + r + n • If sum of numbers of proton , electron and neutron = P , is even • must be symmetric and only s is allowed • If P is odd, antisymmetric- a is allowed

• Symmetry of on exchange will depend on whether exchange of nuclei 0 r electrons or both • If only nuclei exchanged – effects of electron exchange not considered – but effect of nuclear exchange on e can be considered • r is characterised by quantum number J , each J value is associated with (2 J + 1) function

• r is symmetric if J is even • antisymmetric if J is odd • r is a function of angles and π • Nuclear exchange means changing to ( -π) and to ( +π) • Hydrogen has odd number of particles so antisymmetric

• n depends on inter nuclear separation so nuclear exchange has no effect • n is therefore symmetric • It hydrogen in electronic ground state e has symmetric g+ , • + means function is symmetric and symmetric w. r. t inversion as given by g • So over all wave function is symmetric

• If H-H is assumed as A-B, each has spin+1/2 or 1/2 • The possible combination is • A(+)B(+) + B(+)A(+) = A(+)B(+) • A(-)B(-) + B(-)A(-) = A(-)B(-) • A(+)B(-) + B(+)A(-) • A(-)B(+) + B(-)A(+) both 3 &4 are similar • These are symmetric nuclear spin function and has three different function.

A(+)B(-) - B(+)A(+) = 0 A(-)B(-) - B(-)A(-) = 0 A(+)B(-) - B(+)A(-)B(+) - A(-)B(+) both 3 &4 are similar – these are antisymmetric and has only one function • 1 & 2 are excluded • •

• So number of symmetric wave function is 3 times more than antisymmetric wave function • In general – homo nuclear diatomic – of spin ‘i’ – ratio of symmetric to antisymmetric spin function is (i+1) : I • In general – one with greater value is ortho and the other is para

• It is possible to find combinations of wave function which make total either symmetric or antisymmetric • e, v and t are symmetric – so overall symmetry determined by products of r x n • For hydrogen total wave function must be antisymmetric since ‘P’ is odd • total to be antisymmetric either r or n must be antisymmetric but not both

• • • r symmetric J is even n must be antisymmetric(s=0) r antisymmetric J odd n symmetric (s=1) For pare J is even, s=0 Ortho J is odd , s= 1

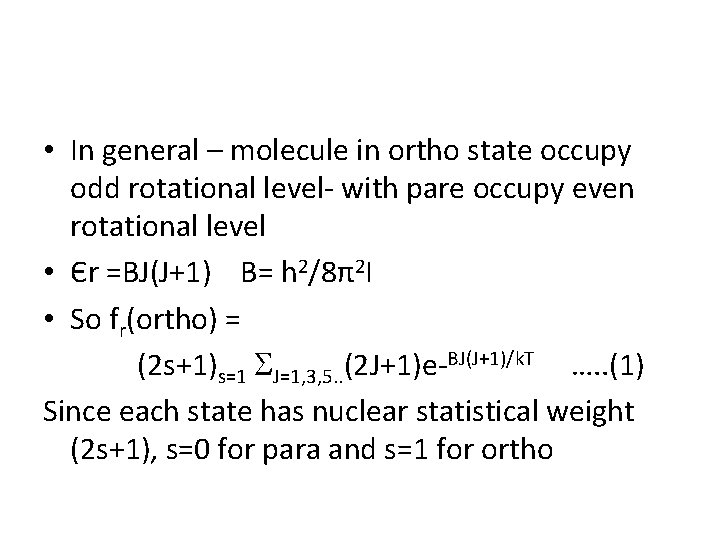
• In general – molecule in ortho state occupy odd rotational level- with pare occupy even rotational level • Єr =BJ(J+1) B= h 2/8π2 I • So fr(ortho) = (2 s+1)s=1 J=1, 3, 5. . (2 J+1)e-BJ(J+1)/k. T …. . (1) Since each state has nuclear statistical weight (2 s+1), s=0 for para and s=1 for ortho

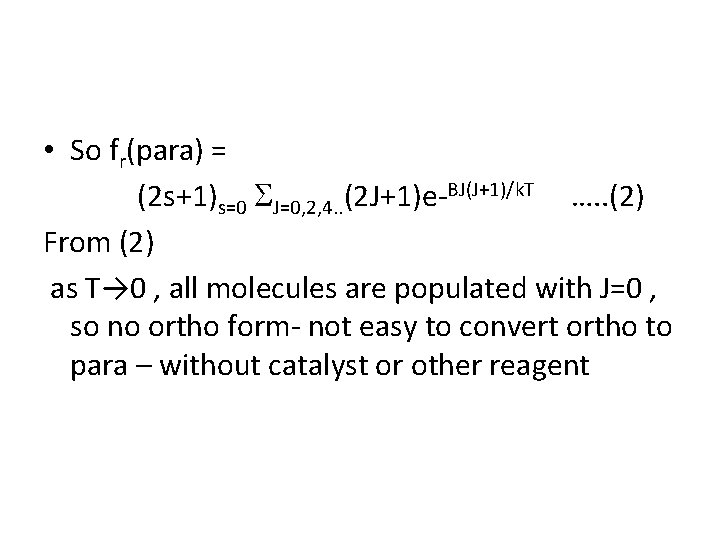
• So fr(para) = (2 s+1)s=0 J=0, 2, 4. . (2 J+1)e-BJ(J+1)/k. T …. . (2) From (2) as T→ 0 , all molecules are populated with J=0 , so no ortho form- not easy to convert ortho to para – without catalyst or other reagent

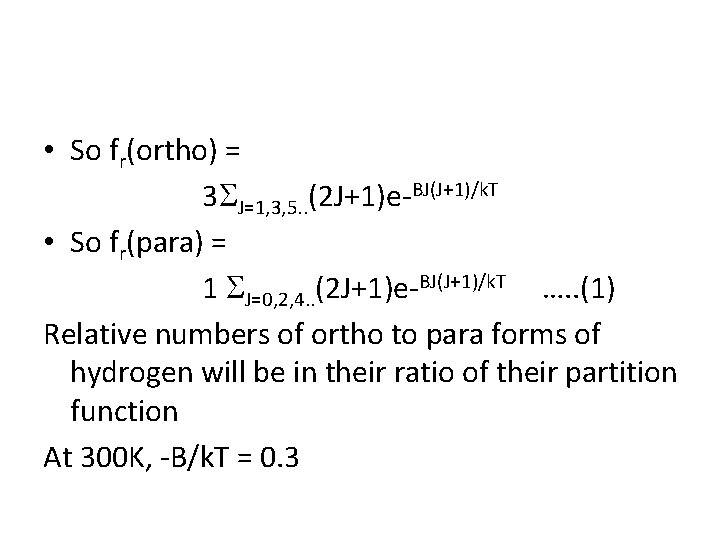
• So fr(ortho) = 3 J=1, 3, 5. . (2 J+1)e-BJ(J+1)/k. T • So fr(para) = 1 J=0, 2, 4. . (2 J+1)e-BJ(J+1)/k. T …. . (1) Relative numbers of ortho to para forms of hydrogen will be in their ratio of their partition function At 300 K, -B/k. T = 0. 3

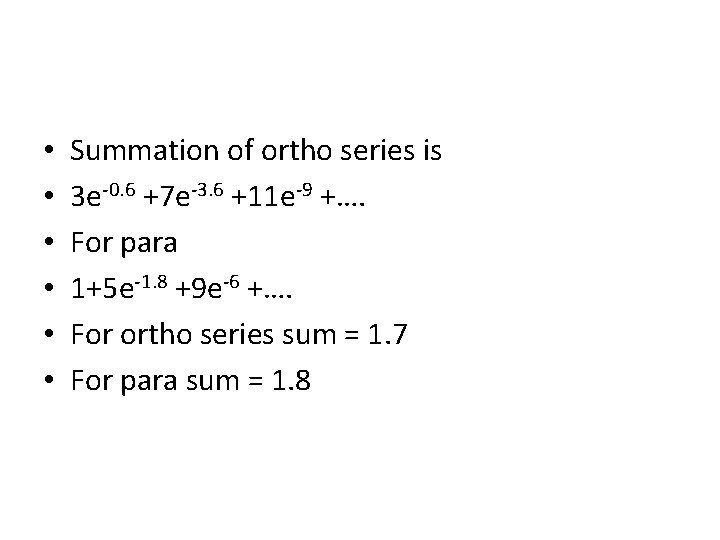
• • • Summation of ortho series is 3 e-0. 6 +7 e-3. 6 +11 e-9 +…. For para 1+5 e-1. 8 +9 e-6 +…. For ortho series sum = 1. 7 For para sum = 1. 8

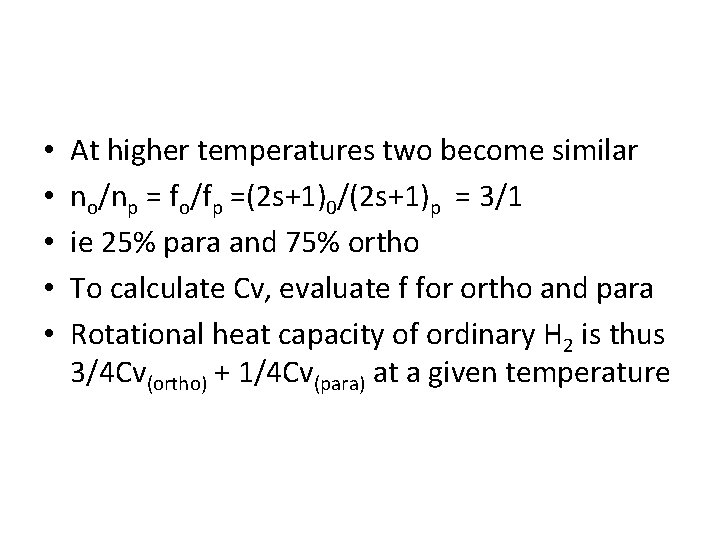
• • • At higher temperatures two become similar no/np = fo/fp =(2 s+1)0/(2 s+1)p = 3/1 ie 25% para and 75% ortho To calculate Cv, evaluate f for ortho and para Rotational heat capacity of ordinary H 2 is thus 3/4 Cv(ortho) + 1/4 Cv(para) at a given temperature

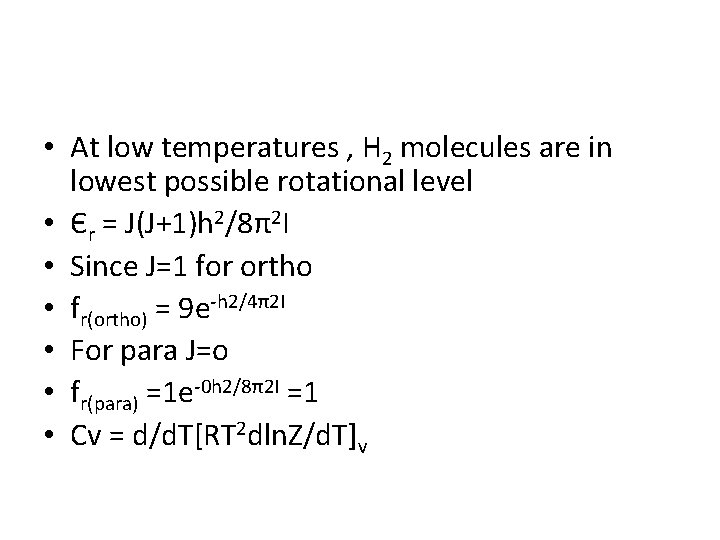
• At low temperatures , H 2 molecules are in lowest possible rotational level • Єr = J(J+1)h 2/8π2 I • Since J=1 for ortho • fr(ortho) = 9 e-h 2/4π2 I • For para J=o • fr(para) =1 e-0 h 2/8π2 I =1 • Cv = d/d. T[RT 2 dln. Z/d. T]v
![dd TLk T 2 dlnfd T Since lnf constant Cvortho 0 = d/d. T[Lk. T 2 dlnf/d. T] Since lnf = constant Cv(ortho) = 0](https://slidetodoc.com/presentation_image_h2/92bf35ab564e58e8759073ca3ca843e4/image-23.jpg)
= d/d. T[Lk. T 2 dlnf/d. T] Since lnf = constant Cv(ortho) = 0 Cv(para) = 0 Rotational contribution to heat capacity is zero at low temperature • As temperature is raised rotational contribution becomes significance and specific heat increases. • • •