Role of Statistics in Model Informed Drug Discovery







- Slides: 38

Role of Statistics in Model Informed Drug Discovery and Development Vlad Dragalin, Ph. D VP Scientific Fellow, Quantitative Sciences Head of QS Consulting and ACT Co. E ASA PT Chapter Spring Symposium| Princeton | May 27, 2016

Overview: What, Why, and How? 1 OUTLINE 4 2 Trial Designs 3 Data Analysis Methods Quantitative Decision Criteria 2

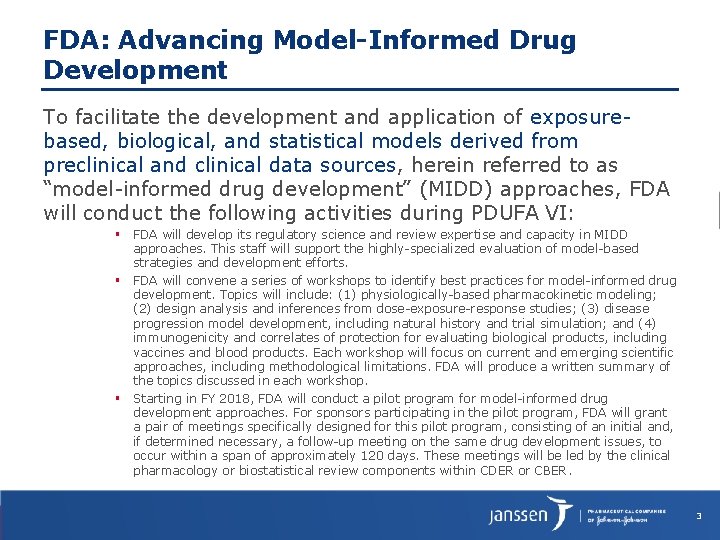
FDA: Advancing Model-Informed Drug Development To facilitate the development and application of exposurebased, biological, and statistical models derived from preclinical and clinical data sources, herein referred to as “model-informed drug development” (MIDD) approaches, FDA will conduct the following activities during PDUFA VI: FDA will develop its regulatory science and review expertise and capacity in MIDD approaches. This staff will support the highly-specialized evaluation of model-based strategies and development efforts. FDA will convene a series of workshops to identify best practices for model-informed drug development. Topics will include: (1) physiologically-based pharmacokinetic modeling; (2) design analysis and inferences from dose-exposure-response studies; (3) disease progression model development, including natural history and trial simulation; and (4) immunogenicity and correlates of protection for evaluating biological products, including vaccines and blood products. Each workshop will focus on current and emerging scientific approaches, including methodological limitations. FDA will produce a written summary of the topics discussed in each workshop. Starting in FY 2018, FDA will conduct a pilot program for model-informed drug development approaches. For sponsors participating in the pilot program, FDA will grant a pair of meetings specifically designed for this pilot program, consisting of an initial and, if determined necessary, a follow-up meeting on the same drug development issues, to occur within a span of approximately 120 days. These meetings will be led by the clinical pharmacology or biostatistical review components within CDER or CBER. 3

EFPIA MID 3 Workgroup 4

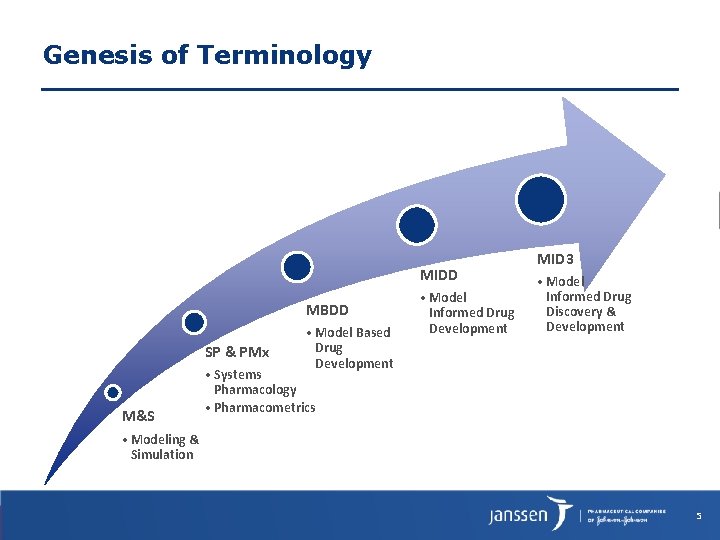
Genesis of Terminology MIDD MBDD SP & PMx M&S • Model Based Drug Development • Model Informed Drug Development MID 3 • Model Informed Drug Discovery & Development • Systems Pharmacology • Pharmacometrics • Modeling & Simulation 5

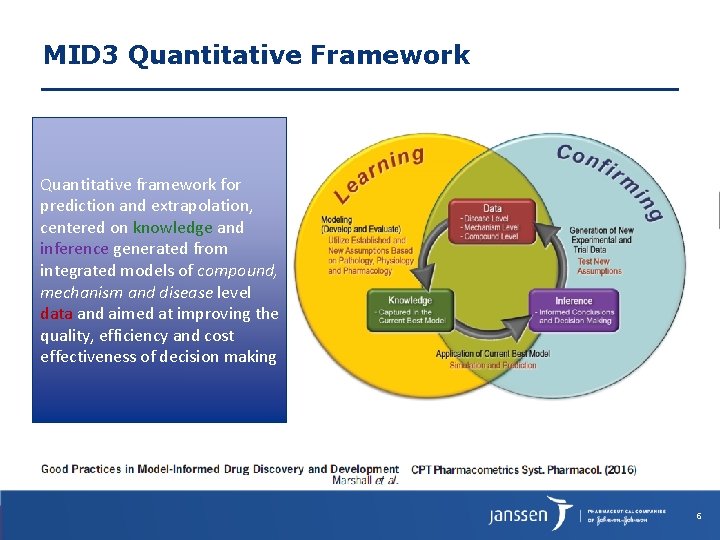
MID 3 Quantitative Framework Quantitative framework for prediction and extrapolation, centered on knowledge and inference generated from integrated models of compound, mechanism and disease level data and aimed at improving the quality, efficiency and cost effectiveness of decision making 6

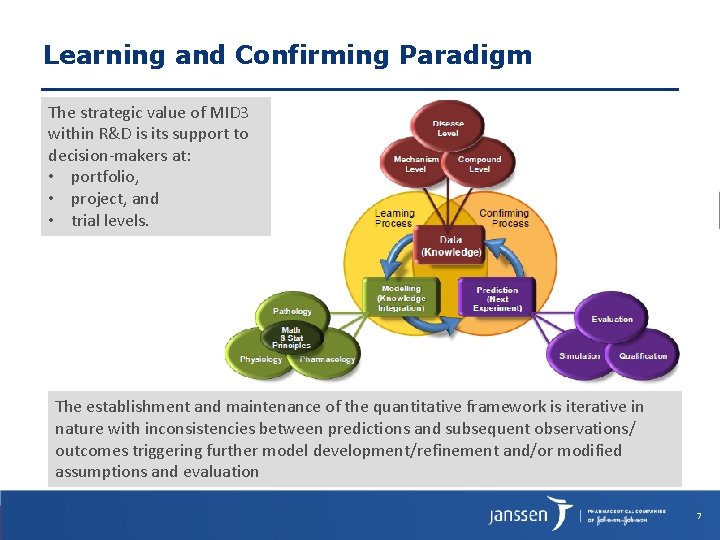
Learning and Confirming Paradigm The strategic value of MID 3 within R&D is its support to decision-makers at: • portfolio, • project, and • trial levels. The establishment and maintenance of the quantitative framework is iterative in nature with inconsistencies between predictions and subsequent observations/ outcomes triggering further model development/refinement and/or modified assumptions and evaluation 7

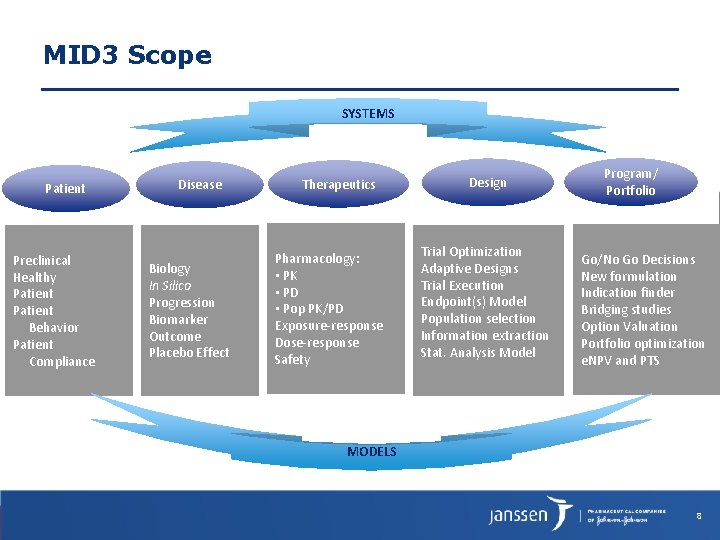
MID 3 Scope SYSTEMS Patient Preclinical Healthy Patient Behavior Patient Compliance Disease Biology In Silico Progression Biomarker Outcome Placebo Effect Therapeutics Pharmacology: • PK • PD • Pop PK/PD Exposure-response Dose-response Safety Design Trial Optimization Adaptive Designs Trial Execution Endpoint(s) Model Population selection Information extraction Stat. Analysis Model Program/ Portfolio Go/No Go Decisions New formulation Indication finder Bridging studies Option Valuation Portfolio optimization e. NPV and PTS MODELS 8

Why MID 3? Need for early development activities to more effectively inform later stage development – “learn and confirm” concept in the late 1990 s A potential solution to the decline in productivity around the turn of the century Strategic component for the FDA Critical Path Initiative and the current Innovative Medicines Initiative, particularly the Drug Disease Model Resources (DDMo. Re) Support regulatory assessment and decision making with respect to trial design, dose and schedule selection, and extrapolation to special populations and label claims 9

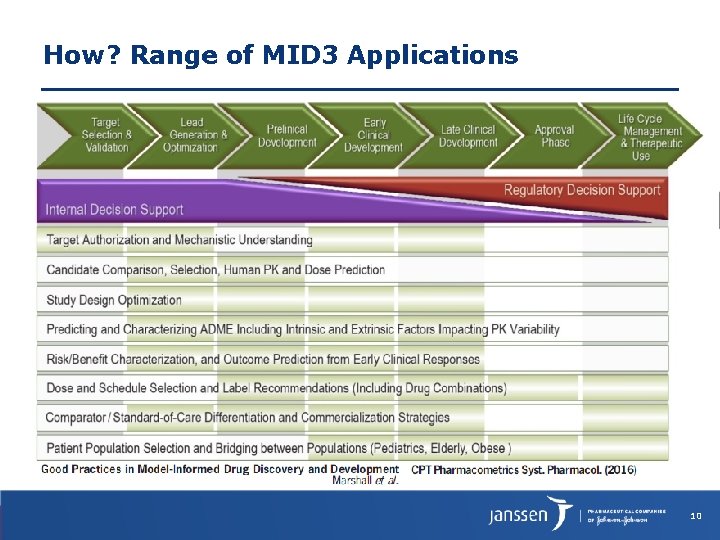
How? Range of MID 3 Applications 10

Potential Benefits MID 3 has been applied across R&D to: increase the confidence in the compound, mechanism, or disease rationales; provide support to internal ‘‘go/no-go decisions’’, dose finding, dose adjustments for patient subgroups; support labelling, benefit-risk, and increasing confidence in next-stage investment; enable more effective decision-making throughout the whole development process of a medical product 11

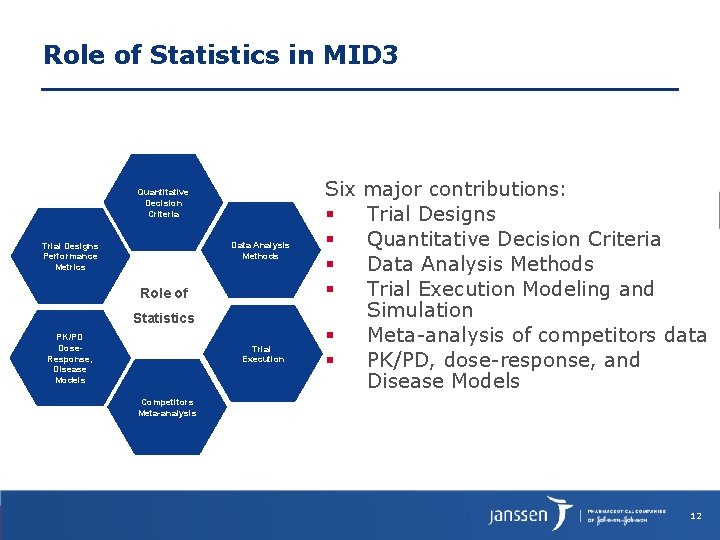
Role of Statistics in MID 3 Quantitative Decision Criteria Data Analysis Methods Trial Designs Performance Metrics Role of Statistics PK/PD Dose. Response, Disease Models Trial Execution Six major contributions: Trial Designs Quantitative Decision Criteria Data Analysis Methods Trial Execution Modeling and Simulation Meta-analysis of competitors data PK/PD, dose-response, and Disease Models Competitors Meta-analysis 12

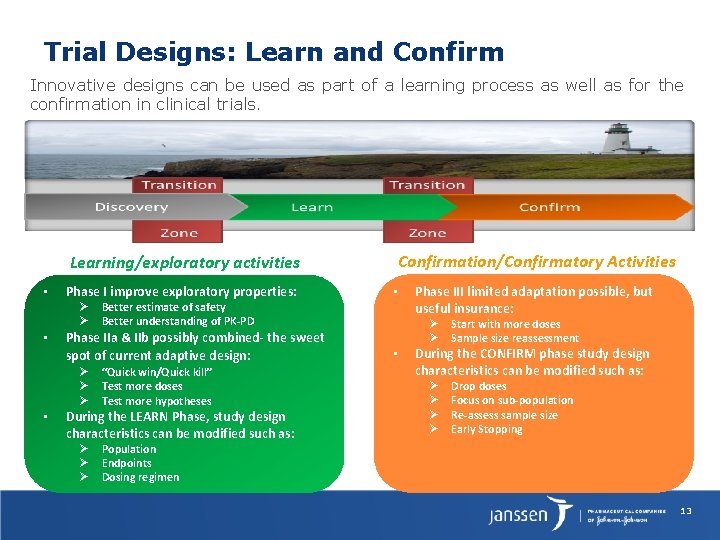
Trial Designs: Learn and Confirm Innovative designs can be used as part of a learning process as well as for the confirmation in clinical trials. Confirmation/Confirmatory Activities Learning/exploratory activities • Phase I improve exploratory properties: Ø Ø • • Better estimate of safety Better understanding of PK-PD Phase IIa & IIb possibly combined- the sweet spot of current adaptive design: Ø Ø Ø “Quick win/Quick kill” Test more doses Test more hypotheses During the LEARN Phase, study design characteristics can be modified such as: Ø Ø Ø • Phase III limited adaptation possible, but useful insurance: Ø Ø • Start with more doses Sample size reassessment During the CONFIRM phase study design characteristics can be modified such as: Ø Ø Drop doses Focus on sub-population Re-assess sample size Early Stopping Population Endpoints Dosing regimen 13

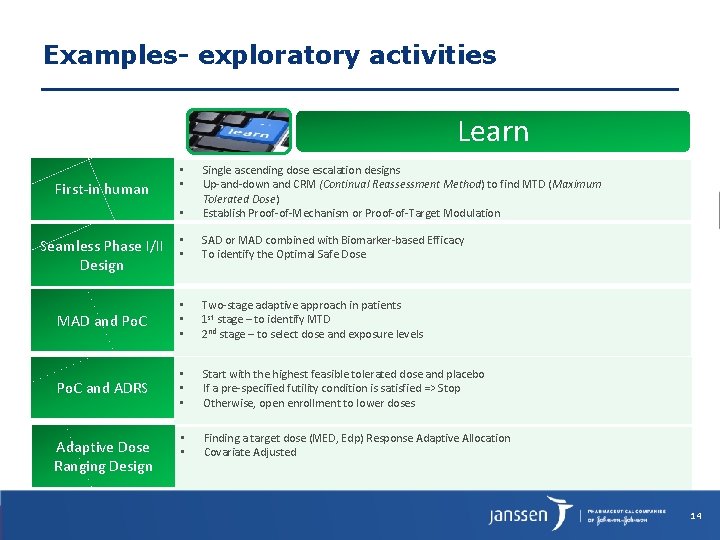
Examples- exploratory activities Learn • Single ascending dose escalation designs Up-and-down and CRM (Continual Reassessment Method) to find MTD (Maximum Tolerated Dose) Establish Proof-of-Mechanism or Proof-of-Target Modulation • • SAD or MAD combined with Biomarker-based Efficacy To identify the Optimal Safe Dose MAD and Po. C • • • Two-stage adaptive approach in patients 1 st stage – to identify MTD 2 nd stage – to select dose and exposure levels Po. C and ADRS • • • Start with the highest feasible tolerated dose and placebo If a pre-specified futility condition is satisfied => Stop Otherwise, open enrollment to lower doses • • Finding a target dose (MED, Edp) Response Adaptive Allocation Covariate Adjusted First-in human Seamless Phase I/II Design Adaptive Dose Ranging Design • • 14

Examples - confirmatory activities Confirm • Sample size adjustment based on blinded or unblinded data: Using nuisance parameter estimate Using treatment effect estimate Adaptive Group Sequential Design • • • Early stopping or efficacy, futility, harm or safety Adjusting the number and/or timing of interim analysis Increasing the maximum sample size Seamless Phase II/III Design • • Design combining the objectives of Phase II dose ranging study and confirmatory Phase III trial in a single protocol Dose selection at the interim analysis Population Enrichment Design • • • Placebo run-in; Active control run-in; Dose titration Adaptive enrichment of the population at the interim analysis Enrich based on biomarker or clinical endpoint response Adaptive Dose Ranging Design • • • Marker by Treatment Design Targeted Design Marker x TRT Design with Responsive adaptive allocation with strata Sample Size Reassessment 15

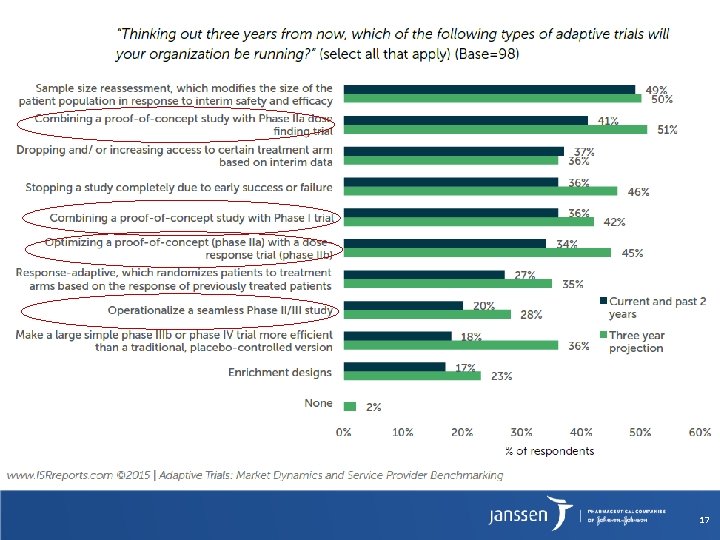
Types of Adaptive Designs Adaptive Design is defined as a multistage study design that uses accumulating data to decide how to modify aspects of the study without undermining the validity and integrity of the trial Trial Level • Number of Subjects • Study Duration Program Level Seamless Adaptive Designs: Combining Conventional Phases in a Single Trial • Treatment Duration Portfolio Level • Compound Finder • Indication Finder • Patient Population • Seamless Phase I/II • Basket Trial • Number of Treatments • MAD and POC • Umbrella Trial • Randomization Ratio • POC and ADRS (Adaptive Dose Ranging Studies) • Platform Trial • Number of Interim Analyses • Seamless Phase II/III 16

17

Trial Designs: Summary Adaptive designs enable more effective decisionmaking throughout the whole development process of a medical product Adaptive designs assist and enhance the decision on which compound to develop for which population The adoption of an adaptive design strategy across the drug development process brings a number of important benefits: – increased R&D efficiency, – increased R&D productivity, – increased probability of success at phase III 18

Data Analysis Methods For Confirm – Pairwise comparisons, ANOVA, ANCOVA – Minimal assumptions: randomization, double-blind, non-informative drop-out For Learn – – Regression model: linear, non-linear, fixed/mixed effects Dose-concentration-response modeling Adaptive randomization Stronger assumptions: DR shape, longitudinal model 19

Dose-Ranging Studies Detecting dose-response – Is there any evidence of activity associated with the drug? – a change in clinical response resulting from a change in dose (Po. C) Identifying clinical relevance – If Po. C is established, determine if a pre-defined clinically relevant response can be obtained within the observed dose range Selecting a target dose – What dose should be selected for Confirm? Estimating the dose response – What is the dose-response profile within the observed dose range? 20

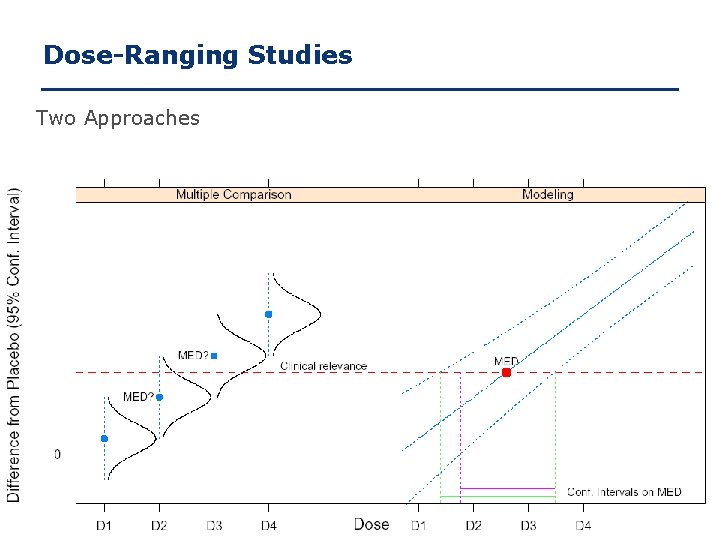
Dose-Ranging Studies Two Approaches 21

Dose-Ranging Studies Multiple Comparison Advantages: – Controls error rate of wrong Po. C decision – Few prior assumptions, little knowledge of dose response shape required, robust Disadvantages: – Restricted to observed dose levels – No margin of error in target dose estimate – Covariates, unbalanced sample sizes, unequally spaced doses ? Model-based Advantages: – Provides CI on target dose estimate – Controls – Accommodates clinical/ regulatory requirements – Better information on target dose Disadvantages: – Strong assumption on dose-response shape – Much more sensitive to model misspecifications 22

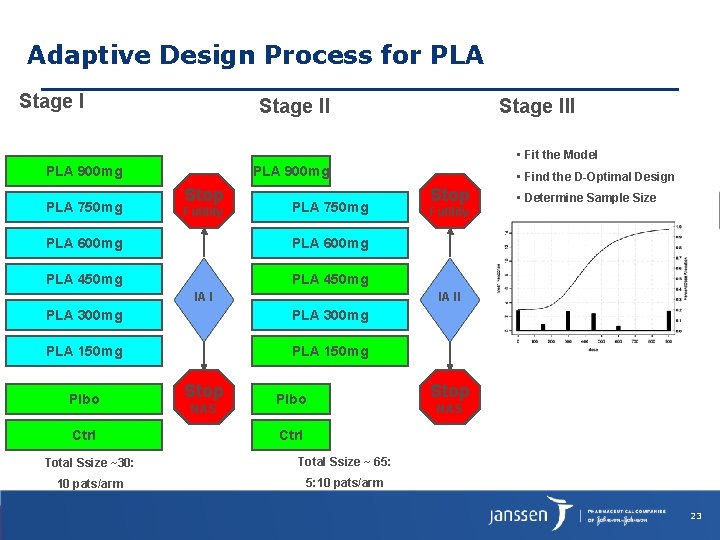
Adaptive Design Process for PLA Stage III • Fit the Model PLA 900 mg PLA 750 mg PLA 900 mg Stop Futility PLA 750 mg PLA 600 mg PLA 450 mg IA I PLA 300 mg PLA 150 mg Ctrl Stop NAS Stop • Determine Sample Size Futility IA II PLA 300 mg Plbo • Find the D-Optimal Design Stop Plbo NAS Ctrl Total Ssize ~30: Total Ssize ~ 65: 10 pats/arm 5: 10 pats/arm 23

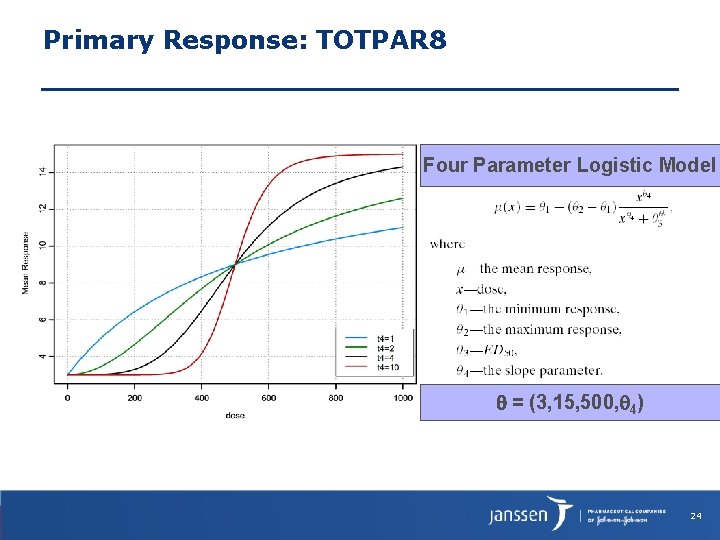
Primary Response: TOTPAR 8 Four Parameter Logistic Model = (3, 15, 500, 4) 24

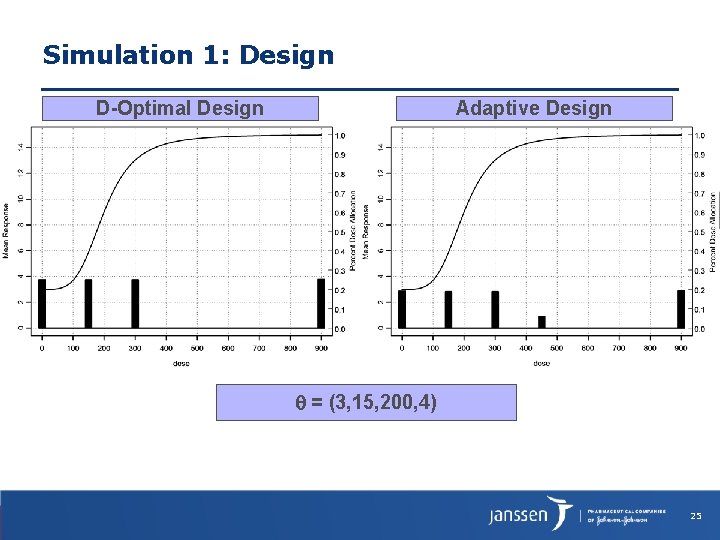
Simulation 1: Design D-Optimal Design Adaptive Design = (3, 15, 200, 4) 25

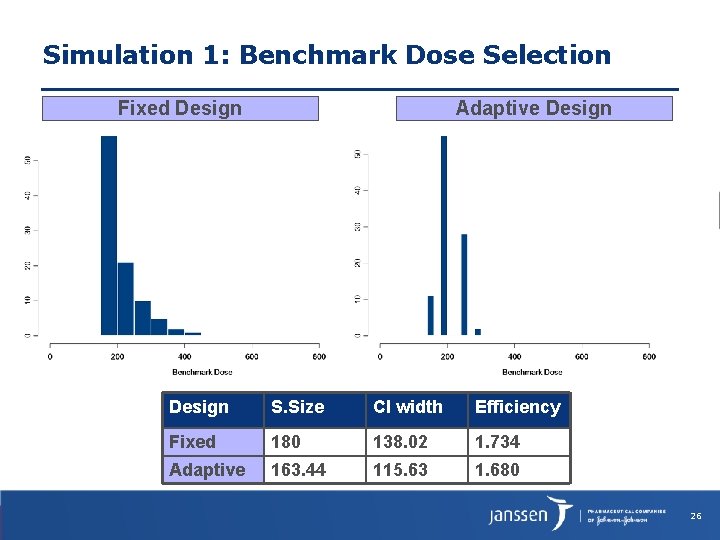
Simulation 1: Benchmark Dose Selection Fixed Design Adaptive Design S. Size CI width Efficiency Fixed 180 138. 02 1. 734 Adaptive 163. 44 115. 63 1. 680 26

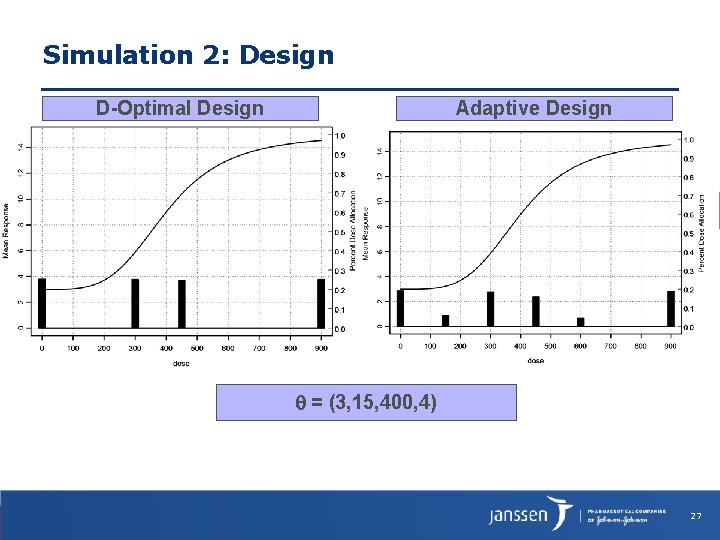
Simulation 2: Design D-Optimal Design Adaptive Design = (3, 15, 400, 4) 27

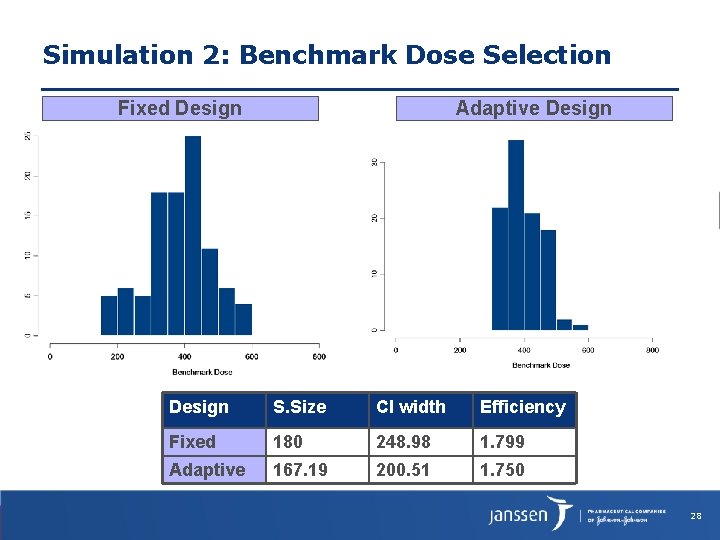
Simulation 2: Benchmark Dose Selection Fixed Design Adaptive Design S. Size CI width Efficiency Fixed 180 248. 98 1. 799 Adaptive 167. 19 200. 51 1. 750 28

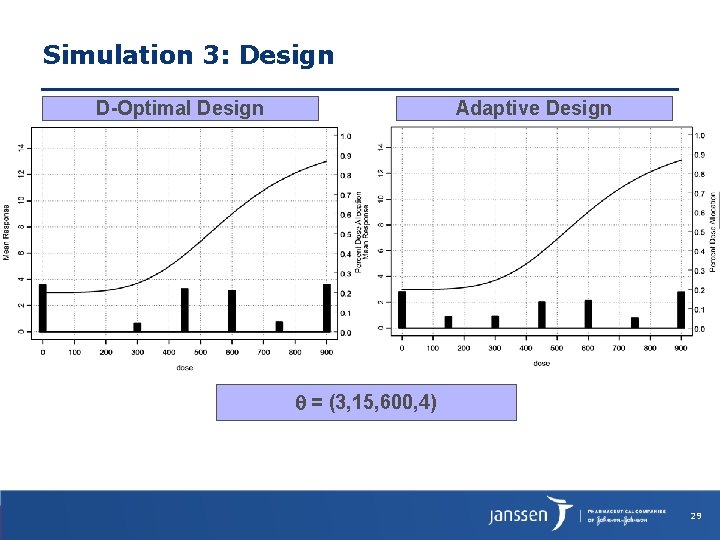
Simulation 3: Design D-Optimal Design Adaptive Design = (3, 15, 600, 4) 29

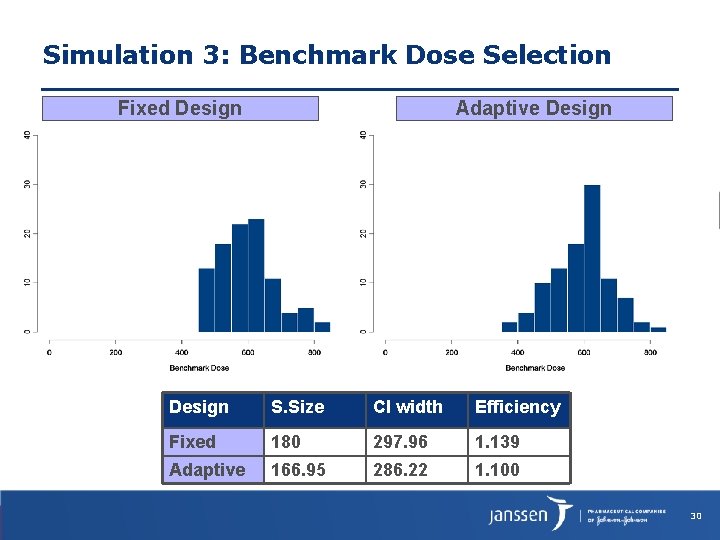
Simulation 3: Benchmark Dose Selection Fixed Design Adaptive Design S. Size CI width Efficiency Fixed 180 297. 96 1. 139 Adaptive 166. 95 286. 22 1. 100 30

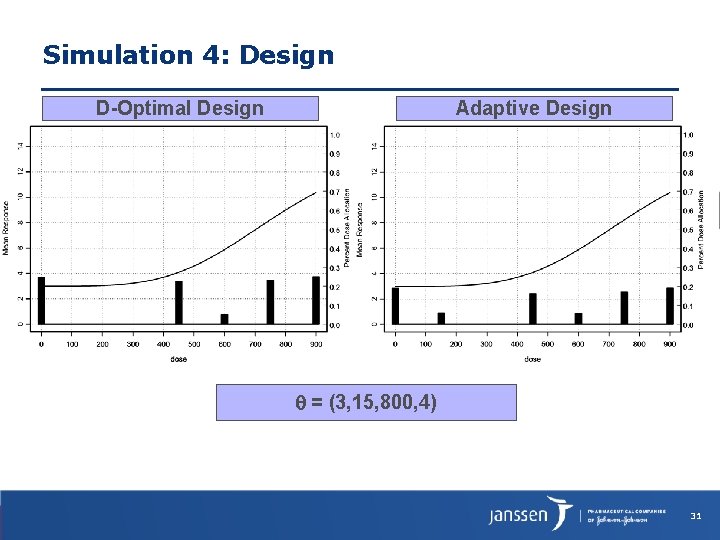
Simulation 4: Design D-Optimal Design Adaptive Design = (3, 15, 800, 4) 31

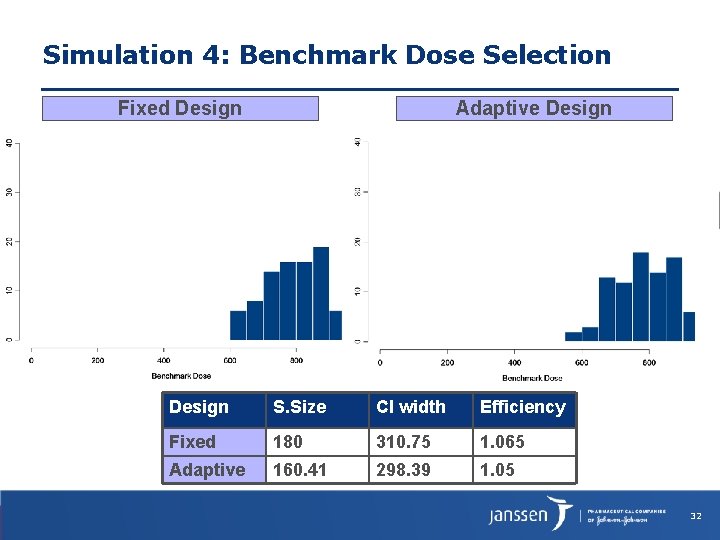
Simulation 4: Benchmark Dose Selection Fixed Design Adaptive Design S. Size CI width Efficiency Fixed 180 310. 75 1. 065 Adaptive 160. 41 298. 39 1. 05 32

Design and Data Analysis Methods: Summary Key challenge in Learn is learning to embrace assumptionrich nonlinear models, avoiding ‘mini-Phase III’ mentality Reliance on empirical, assumption-poor models can be costly, both in the size of trials and the accuracy of decisions made Learn questions may require different study objectives, designs, analysis approaches than Confirm questions 33

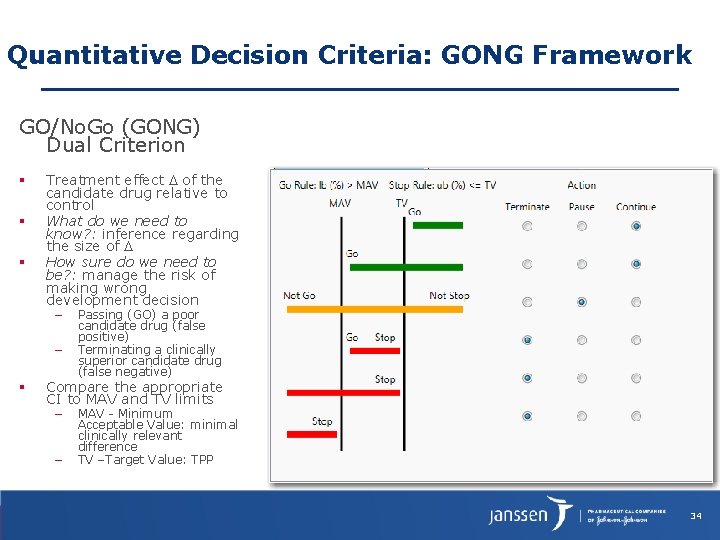
Quantitative Decision Criteria: GONG Framework GO/No. Go (GONG) Dual Criterion Treatment effect of the candidate drug relative to control What do we need to know? : inference regarding the size of How sure do we need to be? : manage the risk of making wrong development decision – – Passing (GO) a poor candidate drug (false positive) Terminating a clinically superior candidate drug (false negative) Compare the appropriate CI to MAV and TV limits – – MAV - Minimum Acceptable Value: minimal clinically relevant difference TV –Target Value: TPP 34

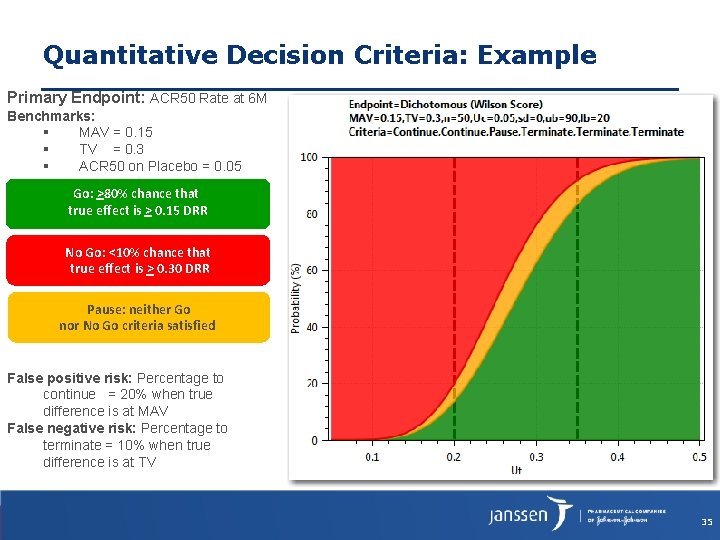
Quantitative Decision Criteria: Example Primary Endpoint: ACR 50 Rate at 6 M Benchmarks: MAV = 0. 15 TV = 0. 3 ACR 50 on Placebo = 0. 05 Go: >80% chance that true effect is > 0. 15 DRR No Go: <10% chance that true effect is > 0. 30 DRR Pause: neither Go nor No Go criteria satisfied False positive risk: Percentage to continue = 20% when true difference is at MAV False negative risk: Percentage to terminate = 10% when true difference is at TV 35

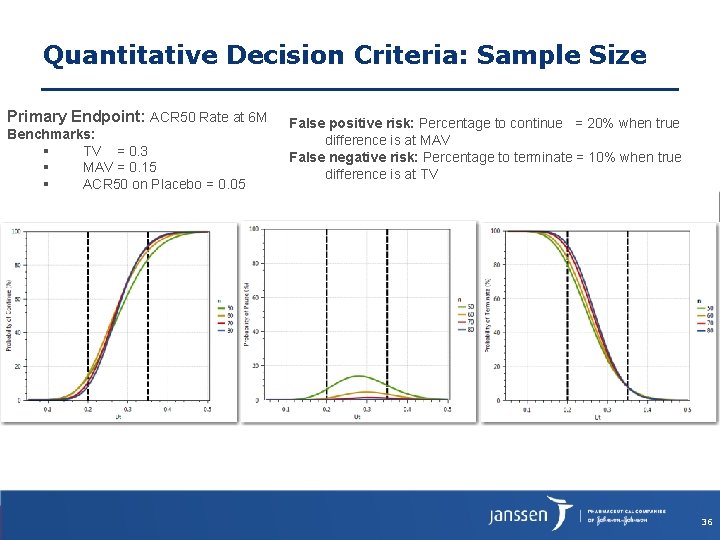
Quantitative Decision Criteria: Sample Size Primary Endpoint: ACR 50 Rate at 6 M Benchmarks: TV = 0. 3 MAV = 0. 15 ACR 50 on Placebo = 0. 05 False positive risk: Percentage to continue = 20% when true difference is at MAV False negative risk: Percentage to terminate = 10% when true difference is at TV 36

Quantitative Decision Criteria: Summary GONG enables us to apply estimation approaches in Learn trials within a Go/No. Go decision framework GONG facilitates the planning of “Learn” phase trials and links the analysis and the Go/No. Go decisions to the TPP GONG considers – the observed value and the associated confidence interval and their relationship to pre-specified TV and MAV. GONG invites cooperation among team members to align on what the trial needs to demonstrate 37

Thank you vdragali@its. jnj. com May 27, 2016 Julius Caesar Bustamante – Pajaros Artwork from Healing Arts Initiative, a nonprofit organization that inspires healing, growth and learning through access to the arts for the culturally underserved.