Role of decreased androgens in the ovarian response













- Slides: 39

Role of decreased androgens in the ovarian response to stimulation in older women Fertil Steril. 2013 Jan; 99(1): 5 -11 Presented by Hsing-Chun Tsai 2013. 02. 26

Outlines • Part I: Effects of testosterone (T) on preantral and antral follicles • Part II: How to improve ovarian response ? – – – Exogenous testosterone DHEA Aromatase inhibition (AI) LH/HCG Growth hormone (GH) / IGF-I

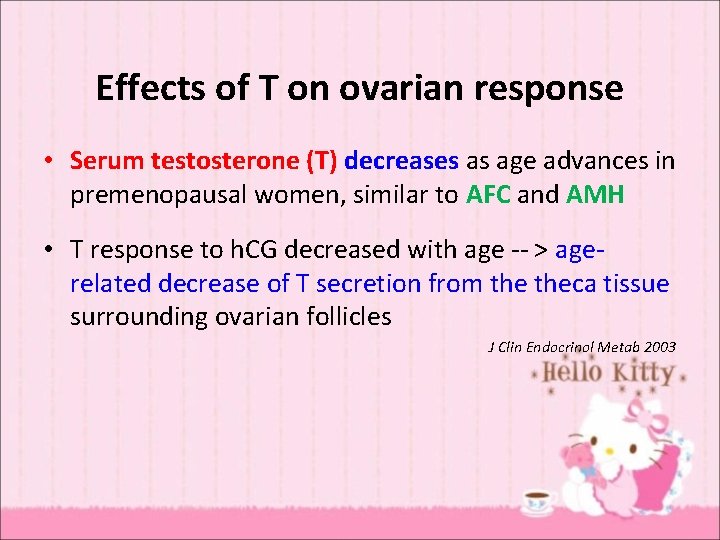
Effects of T on ovarian response • Serum testosterone (T) decreases as age advances in premenopausal women, similar to AFC and AMH • T response to h. CG decreased with age -- > agerelated decrease of T secretion from theca tissue surrounding ovarian follicles J Clin Endocrinol Metab 2003

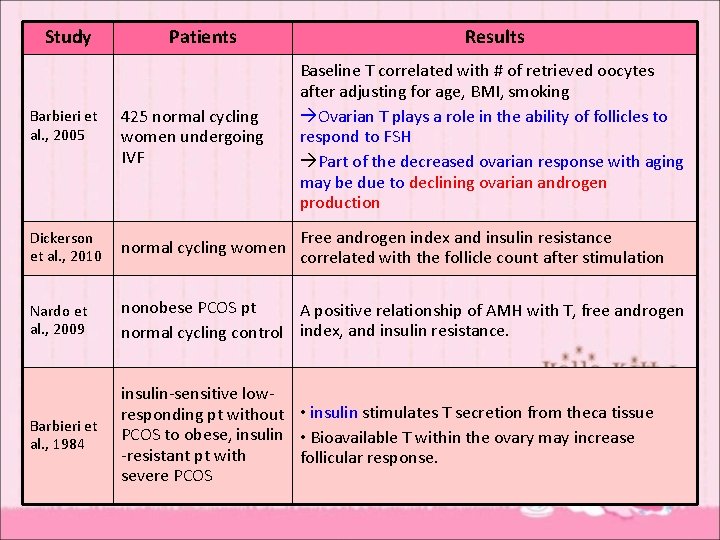
Study Patients Results Barbieri et al. , 2005 425 normal cycling women undergoing IVF Baseline T correlated with # of retrieved oocytes after adjusting for age, BMI, smoking Ovarian T plays a role in the ability of follicles to respond to FSH Part of the decreased ovarian response with aging may be due to declining ovarian androgen production Dickerson et al. , 2010 normal cycling women Free androgen index and insulin resistance correlated with the follicle count after stimulation Nardo et al. , 2009 nonobese PCOS pt A positive relationship of AMH with T, free androgen normal cycling control index, and insulin resistance. Barbieri et al. , 1984 insulin-sensitive lowresponding pt without • insulin stimulates T secretion from theca tissue PCOS to obese, insulin • Bioavailable T within the ovary may increase -resistant pt with follicular response. severe PCOS

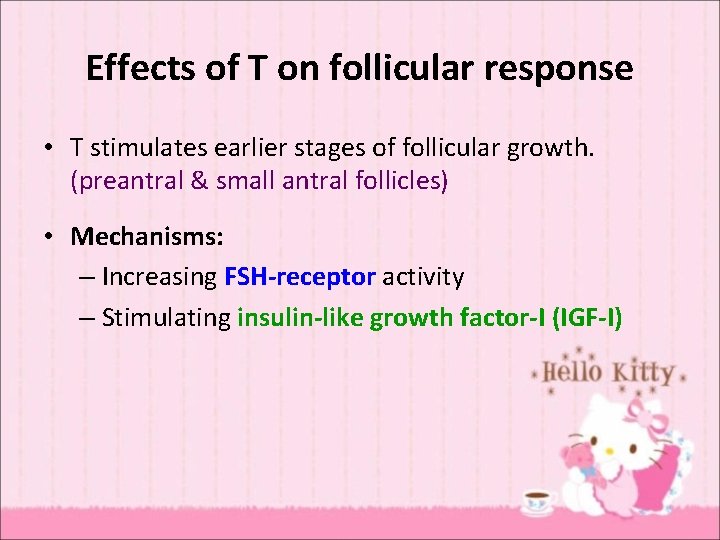
Effects of T on follicular response • T stimulates earlier stages of follicular growth. (preantral & small antral follicles) • Mechanisms: – Increasing FSH-receptor activity – Stimulating insulin-like growth factor-I (IGF-I)

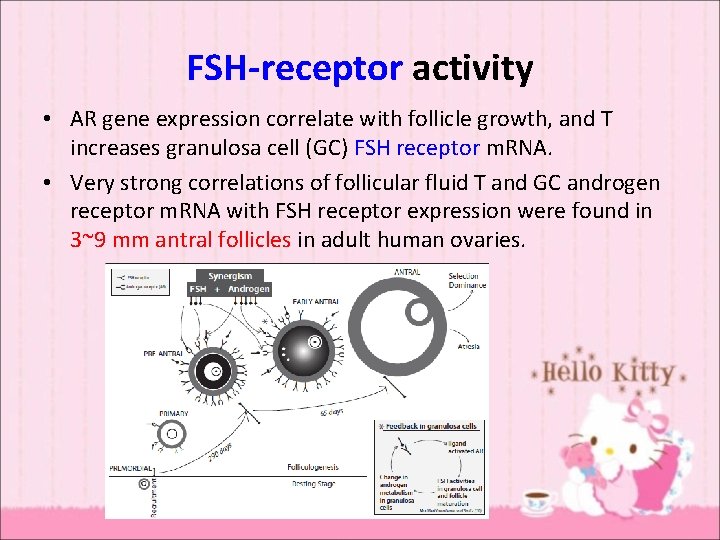
FSH-receptor activity • AR gene expression correlate with follicle growth, and T increases granulosa cell (GC) FSH receptor m. RNA. • Very strong correlations of follicular fluid T and GC androgen receptor m. RNA with FSH receptor expression were found in 3~9 mm antral follicles in adult human ovaries.

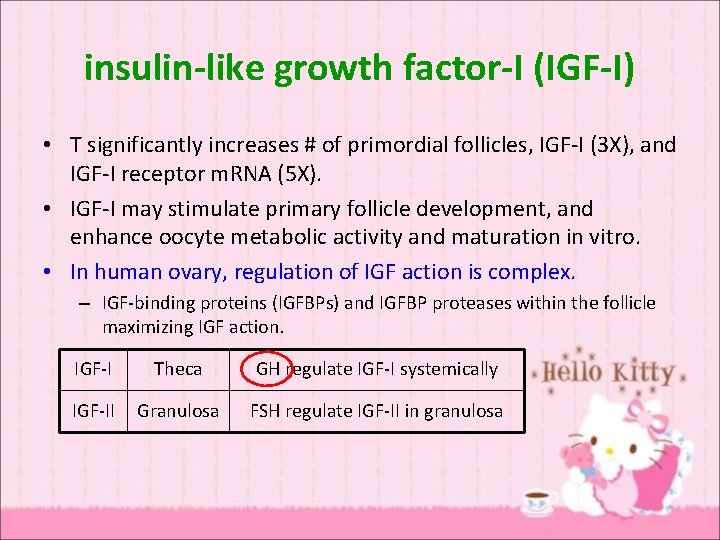
insulin-like growth factor-I (IGF-I) • T significantly increases # of primordial follicles, IGF-I (3 X), and IGF-I receptor m. RNA (5 X). • IGF-I may stimulate primary follicle development, and enhance oocyte metabolic activity and maturation in vitro. • In human ovary, regulation of IGF action is complex. – IGF-binding proteins (IGFBPs) and IGFBP proteases within the follicle maximizing IGF action. IGF-I Theca GH regulate IGF-I systemically IGF-II Granulosa FSH regulate IGF-II in granulosa

Growth hormone • As co-gonadotropin therapy – Action on liver, increasing IGF-I systemically – Secondarily to enhance oocyte maturation, follicle growth and steroidogenesis – No direct action to increase expression of IGF and its receptor genes

Aim • Hypothesis: intraovarian effects of bioavailable T act to cause a continuum of AFC and response from the poor responder to severe PCOS. • Concept: decreasing thecal androgen production due to advancing age causes a progressive impairment of the aging ovary’s ability to respond to stimulation for fertility treatments.

Optimal health patients vs. PCOS with advancing age • Success rate is relatively maintained in PCOS. – oocyte yield falls less with age compared with control – improved oocyte quality • AFC predicts both oocyte quality and quantity. Fertility and Sterility Volume 99, Issue 1 2013 5 - 11

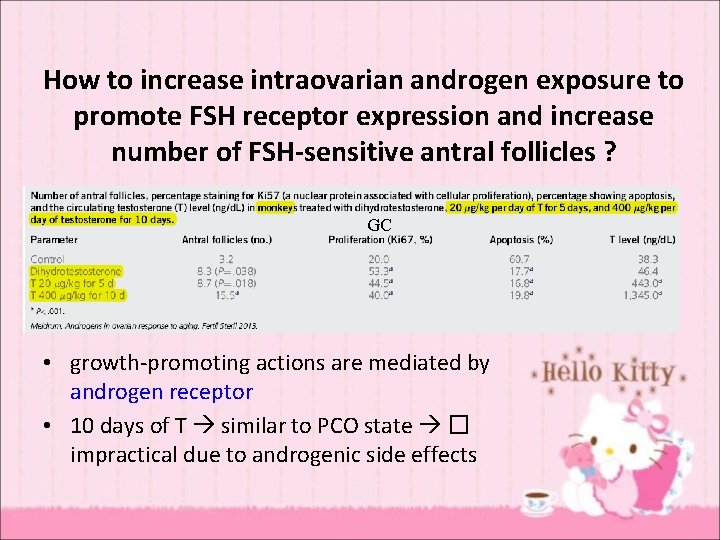
How to increase intraovarian androgen exposure to promote FSH receptor expression and increase number of FSH-sensitive antral follicles ? GC • growth-promoting actions are mediated by androgen receptor • 10 days of T similar to PCO state � impractical due to androgenic side effects

Testosterone


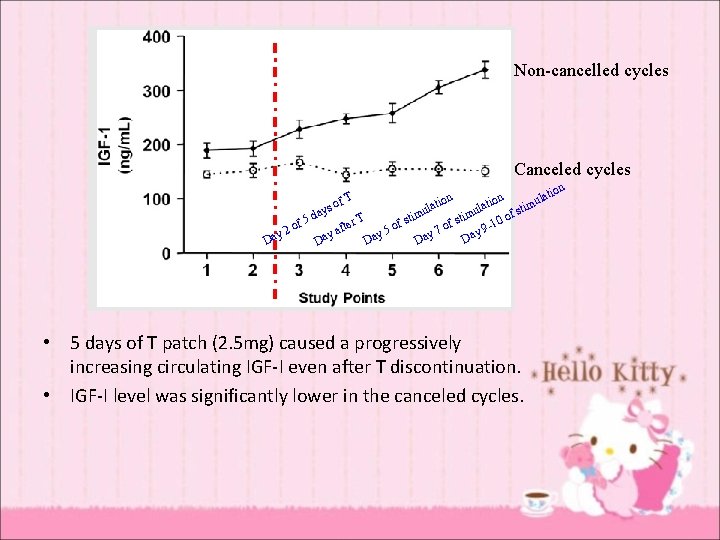
Non-cancelled cycles Canceled cycles on lati atio n l atio imu l s t u u y s f da T stim -10 o r f 5 f f e t o o o f y 9 y 2 y 5 y 7 ya Da Da Da T of • 5 days of T patch (2. 5 mg) caused a progressively increasing circulating IGF-I even after T discontinuation. • IGF-I level was significantly lower in the canceled cycles. n


T

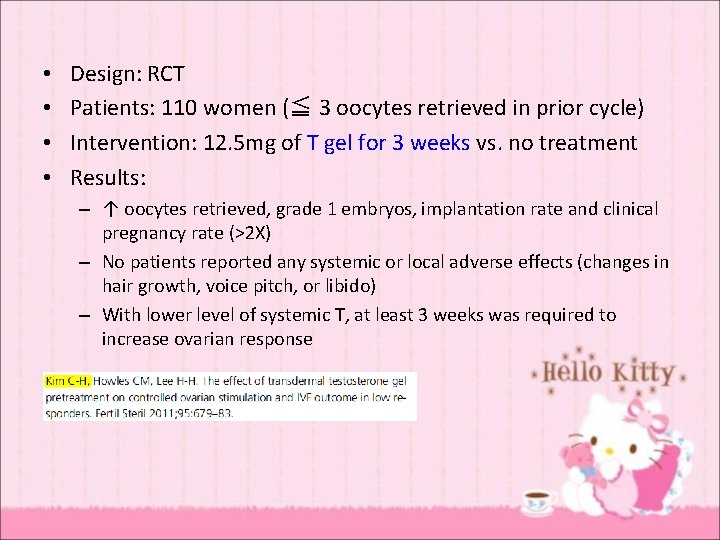
• • Design: RCT Patients: 110 women (≦ 3 oocytes retrieved in prior cycle) Intervention: 12. 5 mg of T gel for 3 weeks vs. no treatment Results: – ↑ oocytes retrieved, grade 1 embryos, implantation rate and clinical pregnancy rate (>2 X) – No patients reported any systemic or local adverse effects (changes in hair growth, voice pitch, or libido) – With lower level of systemic T, at least 3 weeks was required to increase ovarian response

Variables for therapeutic response • Different brand of gel (different levels) • The site and method of application • The duration of treatment • The dose of 12. 5 g for 3 weeks is very close to the threshold producing a response.

DHEA (Dehydroepiandrosterone) • In gonadotropin-stimulated F, almost 50% of follicular fluid T was shown from circulating DHEA sulfate.

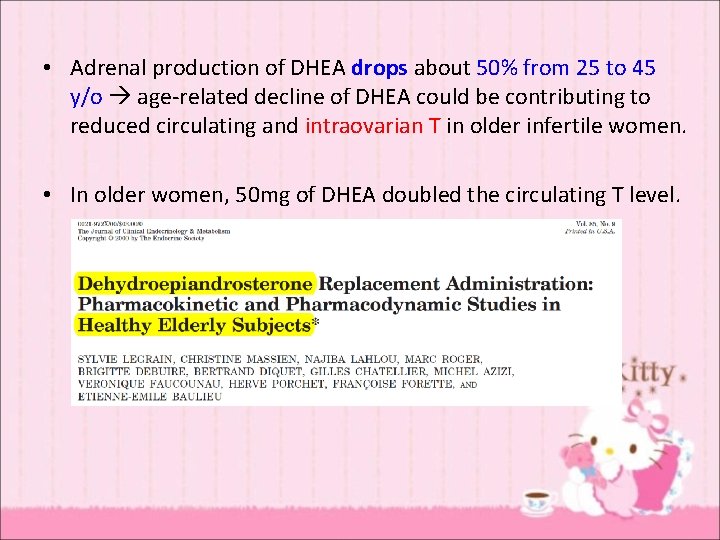
• Adrenal production of DHEA drops about 50% from 25 to 45 y/o age-related decline of DHEA could be contributing to reduced circulating and intraovarian T in older infertile women. • In older women, 50 mg of DHEA doubled the circulating T level.

• Study heterogeneity

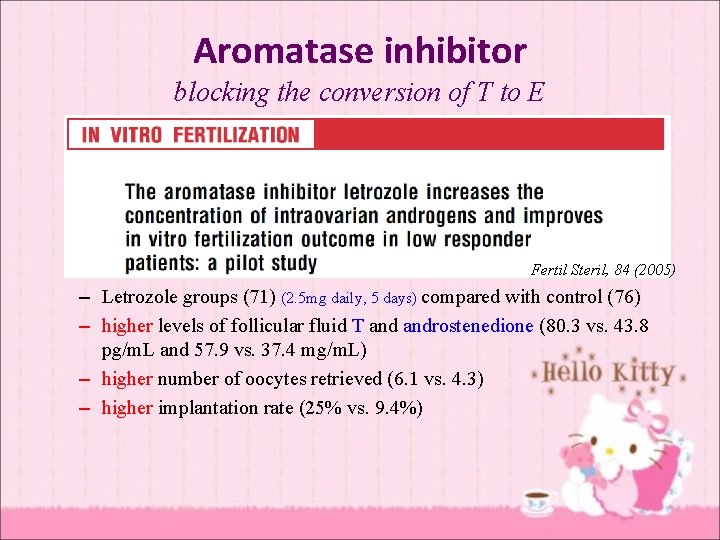
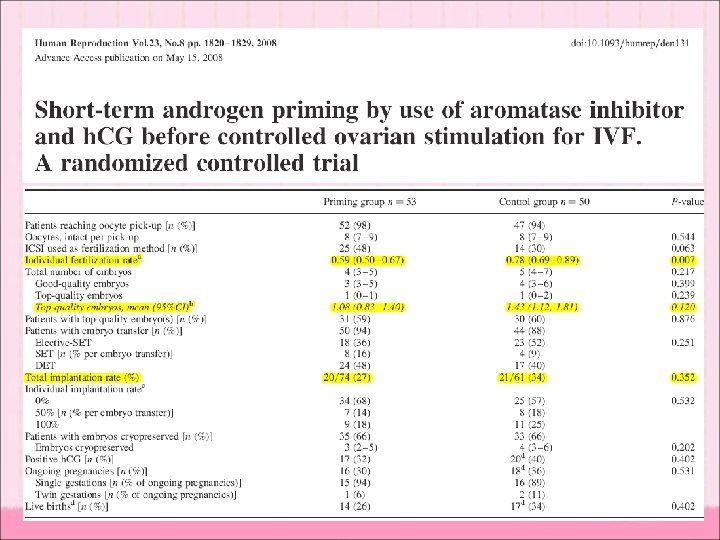
Aromatase inhibitor blocking the conversion of T to E Fertil Steril, 84 (2005) – Letrozole groups (71) (2. 5 mg daily, 5 days) compared with control (76) – higher levels of follicular fluid T androstenedione (80. 3 vs. 43. 8 pg/m. L and 57. 9 vs. 37. 4 mg/m. L) – higher number of oocytes retrieved (6. 1 vs. 4. 3) – higher implantation rate (25% vs. 9. 4%)


LH/HCG • Small daily doses can increase thecal androgen production (insulin, IGF-I) ↔ large doses cause down-regulation of LH/h. CG receptor • ↑ basal LH levels correlated with higher IVF success • Durnerin et al. (2008): long-protocol, daily 300 IU of r. LH for 7 days before stimulation (76) vs. control (71) – Small antral F: 8. 8 vs. 7. 3 (p<. 007) – Fertilized oocytes: 7 vs. 5. 5 (p<. 03) – r-LH in standard IVF showed a possible modest clinical benefit

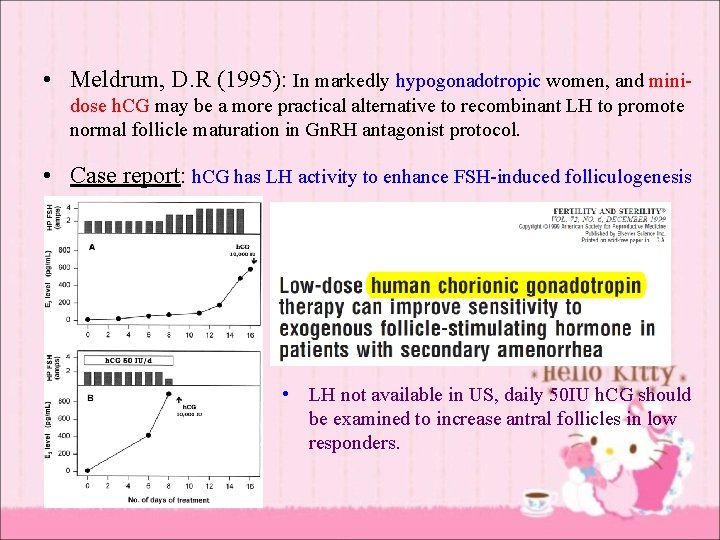
• Meldrum, D. R (1995): In markedly hypogonadotropic women, and minidose h. CG may be a more practical alternative to recombinant LH to promote normal follicle maturation in Gn. RH antagonist protocol. • Case report: h. CG has LH activity to enhance FSH-induced folliculogenesis • LH not available in US, daily 50 IU h. CG should be examined to increase antral follicles in low responders.

Growth hormone/IGF-I • IGF-I synergized with … – FSH inducing GC aromatase activity – LH stimulates thecal androgen production • GH before initiation of ovarian stimulation would be expected to improve the ovarian response.

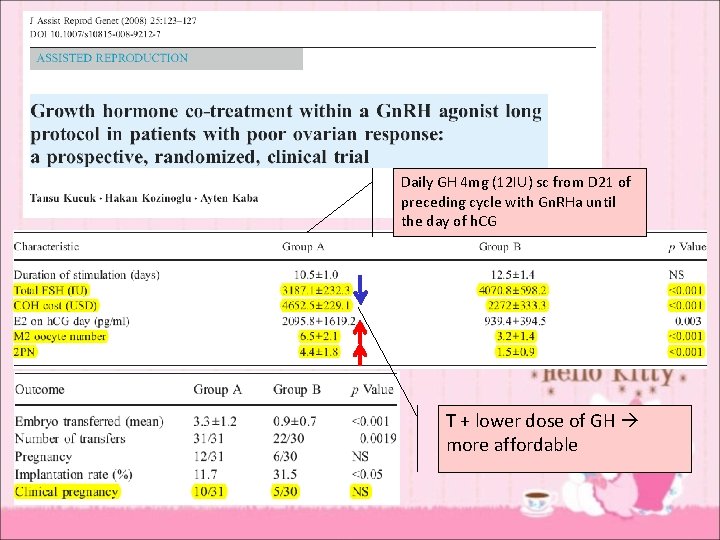
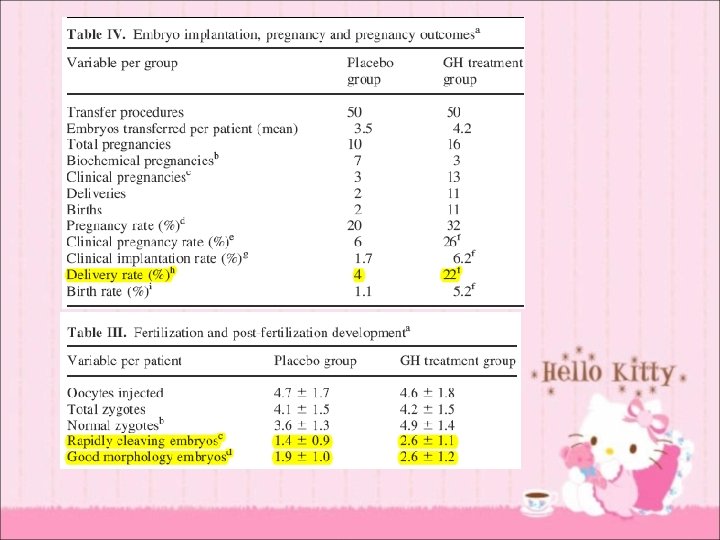
Daily GH 4 mg (12 IU) sc from D 21 of preceding cycle with Gn. RHa until the day of h. CG T + lower dose of GH more affordable

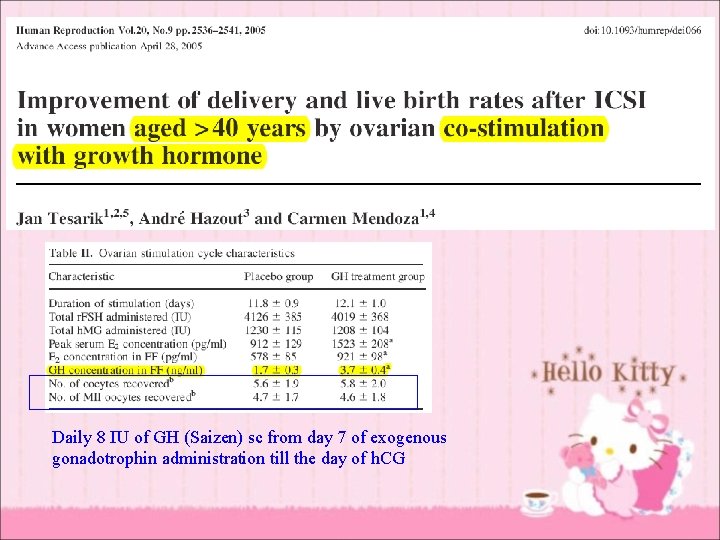
Daily 8 IU of GH (Saizen) sc from day 7 of exogenous gonadotrophin administration till the day of h. CG


• • routine use no difference in outcome & adverse events poor responder significant difference in both live birth rates and pregnancy rates without increasing adverse events (OR 5. 39, 95% CI 1. 89 -15. 35 and OR 3. 28, 95% CI 1. 74 - 6. 20) • heterogeneity

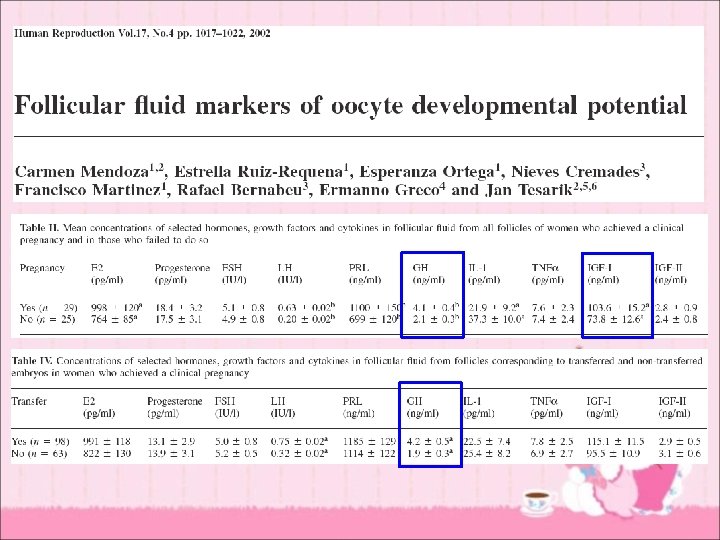
Older women with good ovarian reserve with no response to adequate T IGF-I for mediating T effects on follicle response


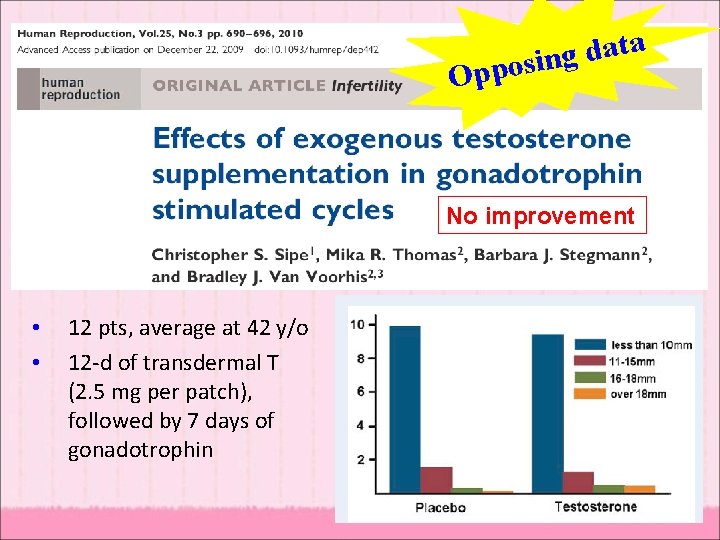
a t a d g pposin O No improvement • • 12 pts, average at 42 y/o 12 -d of transdermal T (2. 5 mg per patch), followed by 7 days of gonadotrophin

Discussion • What we want to know ? – How to explain lower IGF-I levels in poor responders and women failing to conceive with IVF ? (characteristics for older women) – Is lower IGF-I level due to lower circulating GH or reduced hepatic IGF-I production ? – Mechanism of action of IGF-I – Roles of IGF-II, IGFBPs and IGFBP proteases in folliculogenesis

• Age and definitions of poor responder vary among studies. • Whether the intervention increase delivery rates? • Additive effects ? combinations of intervention? ü Low-dose h. CG + letrozole ü T gel + T patch ü + GH during final follicle maturation ovarian T improve ovarian response


• Best widely accepted interventions to improve both response to stimulation and the quality of oocytes and embryos in older women undergoing IVF ü deficient systemic IGF-I level ü intraovarian T levels ü FSH receptor expression

Conclusion • Ovarian testosterone increases the response of antral follicles to stimulation, declines with age, and has effects mediated or potentiated by insulin-like growth hormone I (IGF-I). • T, DHEA, LH/h. CG, AI, GH alone or in combination for enhancing oocyte yield with fertility treatments, particularly in older reproductive-age women

Thank You !