RISK ASSESSMENT DEFINITION A process of evaluation including














- Slides: 15

RISK ASSESSMENT

DEFINITION “A process of evaluation including the identification of the attendant uncertainties, of the likelihood and severity of an adverse effect(s)/event(s) occurring to man or the environment following exposure under defined conditions to a risk source(s)”

TYPES OF RISK ASSESSMENTS ØMicrobiological Risk Assessments ØRisk Assessments in Animal Biosafety ØRisk Assessments of Genetically Modified Plants ØRisk assessments of genetically modified organisms (GMOs)

CRITERIA FOR RISK ASSESSMENT Risk assessments should be performed by the individuals most familiar with the specific characteristics of; The organisms being considered for use The equipment and procedures to be employed Animal models that may be used The containment equipment and facilities available The laboratory director or principal investigator is responsible for ensuring that adequate and timely risk assessments are performed. Once performed, risk assessments should be reviewed routinely and revised when necessary.

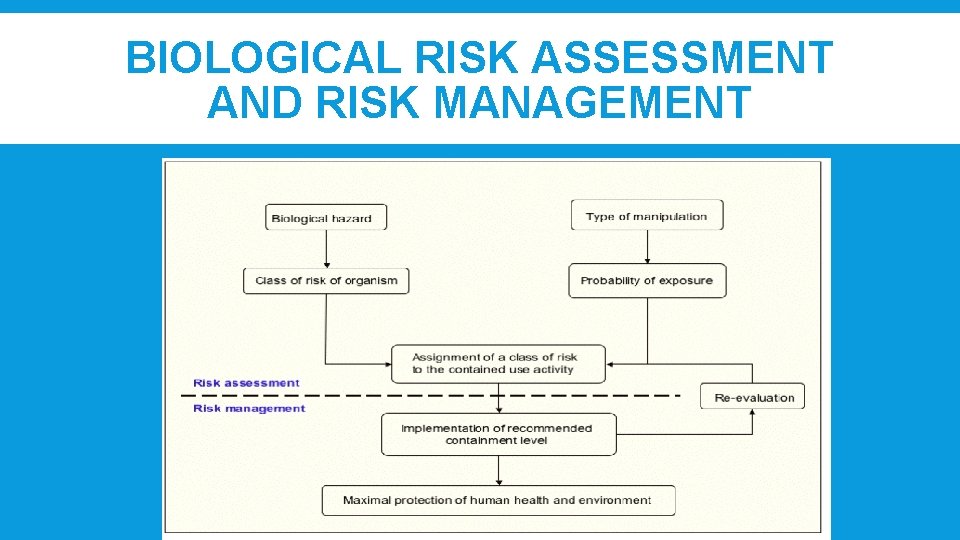
BIOLOGICAL RISK ASSESSMENT AND RISK MANAGEMENT

MICROBIOLOGICAL RISK ASSESSMENT Risk Groups have been assigned to biohazardous agents. They indicate how dangerous a particular bacterium, virus, or other biohazard is. First, determine the risk group of the agent(s) with which you are working. Other factors include; Pathogenicity/Virulence: Is the agent able to infect and cause disease in humans or animals (i. e. , pathogenicity)? What is the degree of disease severity in individuals (i. e. , virulence)? Route of Infection: How does the agent gain entry in to the host (i. e. , ingestion, inhalation, mucous membranes, subcutaneous, genitourinary)? Mode of Transmission: How does the agent travel to the host (e. g. , direct contact, indirect contact, casual contact, aerosolized droplet or airborne transmission, vectors, zoonosis, intermediate host)? Survival in the Environment: How stable is the agent outside the host? Under what environmental conditions can it survive and for how long?

Infectious Dose: What amount of agent is required to cause an infection in the host (measured in number of organisms)? Availability of Effective Preventative and Therapeutic Treatments: Are effective preventative measures available (e. g. , vaccines)? Are effective treatments available (e. g. , antibiotics, antivirals)? Host Range: What are the primary, intermediate, and dead-end hosts? Does the agent cause infection in a wide range of species, or is the host range more restricted?

MICROBIOLOGICAL RISK ASSESSMENT Natural Distribution: Is the agent present in specific area? Is it prevalent in a particular location, region, or human or animal population? Is the agent nonindigenous? Impact of Introduction and/or Release into the Environment or the Public: If the agent were introduced into the population or released into the environment, what would be the economic, clinical, and biosecurity impact? Aerosol Generation: Are equipment or procedures that may generate aerosols being used (e. g. , pipetting, centrifugation, homogenization)? Personnel can be exposed to infectious aerosols by direct inhalation of aerosolized droplets or by ingestion of droplets that settle on surfaces or hands. Concentration of the Pathogen: The concentration of the agent may vary depending on the work being performed (e. g. , diagnostic specimens may contain a lower concentration of pathogen than pure cultures). Either non-pathogenic or attenuated bacterial strains should be used when possible, especially in teaching laboratories. This practice will help reduce the risk of students and/or their family members becoming ill.

MICROBIOLOGICAL RISK ASSESSMENT Severity and duration of illness (acute versus chronic effects) Availability of vaccines or antitoxins Use of chemical safety practices appropriate to the techniques used (i. e. , solvents, acids)

RISK ASSESSMENTS IN ANIMAL BIOSAFETY Risk assessments in case of model animals should be following: üSpecie of the animal should be known and only permissive species should be allowed to be used in the labs. üThe nature of the animals, i. e. their aggressiveness and tendency to bite and scratch should be known. üthat principal investigator (PI) should identified the risks involved, routes of exposure (nature/ lab settings), signs/symptoms of infection , potential for shedding from animals, at risk personnel (those contraindicated from work) and serum banking, immunizations, screening/evaluation. üAn appropriate medical surveillance program for the staff must be instituted. A safety or operations manual must be prepared and adopted.

RISK ASSESSMENTS IN ANIMAL BIOSAFETY üBiohazard warning signs should be posted on doors and other appropriate places. üHeating, ventilation and lighting of the room containing cages must be adequate. If mechanical ventilation is provided, the airflow must be inwards. Exhaust air is discharged to the outside and should not be recirculated to any part of the building. Access must be restricted to authorized persons. üNo animals should be admitted other than those for experimental use. üThere should be an arthropod and rodent control program. üAfter use, work surfaces must be decontaminated with effective disinfectants

RISK ASSESSMENTS IN ANIMAL BIOSAFETY üBiological safety cabinets (Classes I or II) or isolator cages with dedicated air supplies and HEPA-filtered exhaust air must be provided for work that may involve the generation of aerosols. üAnimal bedding materials must be removed in a manner that minimizes the generation of aerosols and dust. All waste materials and bedding must be decontaminated before disposal. üAnimal cages must be decontaminated after use. üAnimal carcasses should be incinerated. üAll injuries, however minor, must be treated appropriately, reported and recorded.

RISK ASSESSMENTS IN ANIMAL BIOSAFETY üThere must be mechanical ventilation to ensure a continuous airflow through all the rooms. Exhaust air must pass through HEPA filters before being discharged to the atmosphere without recirculation. The system must be designed to prevent accidental reverse flow and positive pressurization in any part of the animal house. üThere must be medical supervision of staff. ü Personal assessment factors should be known that include personal protective equipment/clothing and the working area.

RISK ASSESSMENTS OF GENETICALLY MODIFIED PLANTS ØRisk assessment of GM plants starts with problem formulation including hazard identification, hazard characterization, exposure characterization, risk management strategies, and an overall risk evaluation. Risk assessment of genetically modified organisms considers seven specific areas of concern that includes: ØPersistence and invasiveness of the GM plant or its compatible relatives, including plant-to-plant gene transfer. Ø Plant-to-micro-organism gene transfer ØInteraction of the GM plant with target organisms

RISK ASSESSMENTS OF GENETICALLY MODIFIED PLANTS üInteraction of the GM plant with non-target organisms, including criteria for selection of appropriate species and relevant functional groups for risk assessment üImpact of the specific cultivation, management and harvesting techniques; including consideration of the production systems and the receiving environment üEffects on biogeochemical processes üEffects on human and animal health