Riga Nuclear Medicine Centre Vitlijs Skrvelis Head of















![NMC radiopharmaceuticals in 2017 [18 F]-FDG (fluorodeoxyglucose) § § § Breast cancer, Lymphomas Melanoma, NMC radiopharmaceuticals in 2017 [18 F]-FDG (fluorodeoxyglucose) § § § Breast cancer, Lymphomas Melanoma,](https://slidetodoc.com/presentation_image/285dbb20d1be9b70051c2d926bd8ecb4/image-20.jpg)
![Plans for 2018. – 2019. [18 F]-PSMA - for early diagnostic of recurrent prostate Plans for 2018. – 2019. [18 F]-PSMA - for early diagnostic of recurrent prostate](https://slidetodoc.com/presentation_image/285dbb20d1be9b70051c2d926bd8ecb4/image-21.jpg)

- Slides: 23

Riga Nuclear Medicine Centre Vitālijs Skrīvelis Head of R&D, Chairman of the Board vitalijs@rnmc. lv 1

Introduction The Nuclear Medicine Centre (NMC) was founded in 2011 as a private investors and university partnership project with aim to establish in Latvia the cyclotron/PET centre to: § § § provide precise cancer diagnostics in both public and private sector ensure scientific support for invention of new, effective treatments of cancer, neurodegenerative and heart diseases manufacture and distribute PET radiopharmaceuticals in the Baltic & Scandinavia region 2

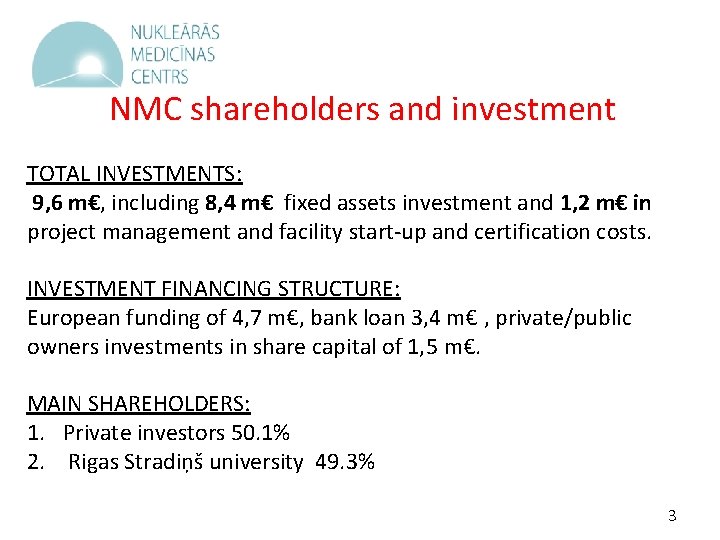
NMC shareholders and investment TOTAL INVESTMENTS: 9, 6 m€, including 8, 4 m€ fixed assets investment and 1, 2 m€ in project management and facility start-up and certification costs. INVESTMENT FINANCING STRUCTURE: European funding of 4, 7 m€, bank loan 3, 4 m€ , private/public owners investments in share capital of 1, 5 m€. MAIN SHAREHOLDERS: 1. Private investors 50. 1% 2. Rigas Stradiņš university 49. 3% 3

Modern radiopharmaceutical manufacturing and PET/CT centre covering 3 interconnected competences: ü GMP pharmaceutical manufacturing ([18 F]-FDG, [68 Ga]-PSMA ) ü Medical institution for visual diagnostic (PET/CT ) ü Research institution (18 Me. V cyclotron) capable of manufacturing at least 6 short life isotopes: C 11, N 13, O 15, F 18, Cu 64, Ga 68 and develope radiopharmaceuticals based on cyclotron solid target system (e. g. 89 Zr) 4

Operational and safety licences 11 licences acquired according to EU legislation § § § 4 for radiation safety – cyclotron, 68 Ge/68 Ga generator, synthesis of 18 F radiopharmaceuticals, running of PET/CT scanner 3 for medicinal activities – medical centre, radio-pharmacy, daily stationary 4 for pharmaceutical manufacturing – manufacturing of APIs, pharma wholesale, manufacturing of sterile radiopharmaceuticals, Good manufacturing Practice (GMP) compliance certificate. NMC building commissioned by construction authority on February 12 th, 2016. PET/CT scans with FDG started in June, 2016; FCH in August, 2016, and Ga-PSMA in November, 2016. 5

Equipment § § § Cyclotron 18 Me. V (produced by IBA, Belgium) for production of radionuclides for PET - 11 C (t 1⁄2 20 min), 13 N (t 1⁄2 10 min), 15 O (t 1⁄2 2 min), 18 F (t 110 min), 64 Cu (t 68 1⁄2 12 h), Ga (t 1⁄2 68 min) 6 «hot-cells» of whom 4 are dedicated for synthesis and 2 for sterile dispensing located in Class C clean rooms for synthesis of sterile radiopharmaceuticals Philips Gemini TF PET/CT scanner with built-in Time-of-Flight technology and 64 -slice Computer Tomography scanner 6

Proud of. . . 7

Cyclotron facility layout 8

Staff experience Ø Nuclear Medicine Radiologists Dr. Marika Kalniņa and Dr. Lilita Roznere trained in Klinikum rechts der Isar Ismaninger (Munich, Germany). Ø Radiation safety experts on site Jāzeps Malnačs and Linda Meistere with more than 10 years of experience. Ø Radiochemist, cyclotron engineer and quality control staff training at ICNAS (Coimbra, Portugal), BIONT (Bratislava, Slovakia) Ø Continious radiologist, resident, radiographer, and production staff training abroad in cooperation with International Atomic Energy Agency (IAEA), Vienna, Austria. 9

Visits of IAEA representatives to NMC ü ü ü 4 December 2015 – Mr. Jing Zhang, SAEA Section Head and pmo Division for Europe, representative of International Atomic Energy Agency 26 March 2015 and 30 August 2013 – Mr. Ivan Videnovic, Programme Management Officer at International Atomic Energy Agency 31 May 2016 – Mr. Yukiya Amano, Director General of the IAEA 10

11

12

13

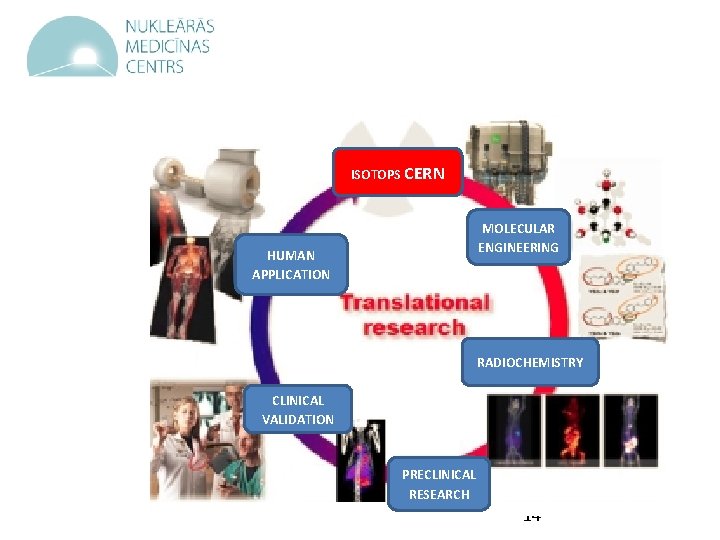
ISOTOPS CERN HUMAN APPLICATION MOLECULAR ENGINEERING RADIOCHEMISTRY CLINICAL VALIDATION PRECLINICAL RESEARCH 14

15

Market potential for Terbium isotops for prostata cancer cure More than 1. 1 million cases of prostate cancer were recorded globaly in 2012, accounting for around 8 per cent of all new cancer cases and 15 per cent in men http: //www. wcrf. org/int/cancer-facts-figures/data-specific-cancers/prostate-cancer-statistics 2 stages to make 155 TB, 149 TB & 161 TB izotops marketable via outlicencing A. Based on CERN-MEDICIS Terbium isotopes develope radiopharmaceuticals to prove clinical superioty against competing radio-tracers already on the market – 68 Ga-PSMA (for PET/CT diagnostic) and 177 Lu-PSMA (for radio-therapy). B. Develope and standartize 30 -70 m. EV cyclotron’s based technology for Terbium: Target, beaming, isotope collection – Isotope purification to the level suitable for GMP compliant synthesis – 16

Riga Stradins university Nuclear medicine centre offer for collaboration NMC own competences: - elaborate and standartise synthesis technology and analytics for radiopharmaceuticals, g. e. 155 Tb-PSMA complex, - GMP compliant manufacturing of 155 TB, 149 TB & 161 TB based sterile radiopharmaceuticals for clinical trials, - GMP compliant manufacturing of clinical comparision radiopharmaceuticals, like 68 Ga-PSMA (for PET/CT diagnostic) and 177 Lu-PSMA (for radio-therapy), In colaboration with research institutes in LV - experiments on laboratory animals (mouse, rat) 17

Statistics 563 patients examined during Jun 2016 – Dec 2017 TYPE OF CANCER Lymphomas % 27 Prostate cancer 17 Breast cancer 15 Uterime, ovarian ~4 Melanoma ~4 ~3 Lung tumors Other (e. g. gastrointestinal, 30 gynecologic, endocrine, colorectal) Efficacy of chemo No 20% Partly 60% Yes 20% Staging Reccurence No 33% Increases 25% Remains the same 75% Yes 67% 18

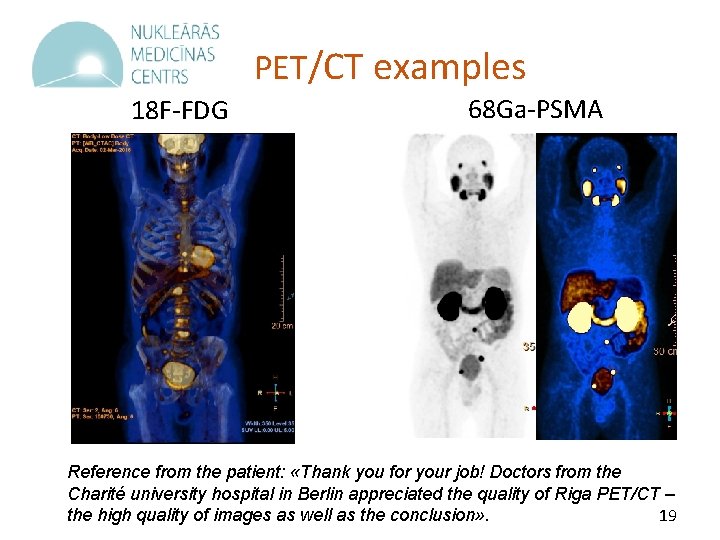
PET/CT examples 18 F-FDG 68 Ga-PSMA Reference from the patient: «Thank you for your job! Doctors from the Charité university hospital in Berlin appreciated the quality of Riga PET/CT – 19 the high quality of images as well as the conclusion» .
![NMC radiopharmaceuticals in 2017 18 FFDG fluorodeoxyglucose Breast cancer Lymphomas Melanoma NMC radiopharmaceuticals in 2017 [18 F]-FDG (fluorodeoxyglucose) § § § Breast cancer, Lymphomas Melanoma,](https://slidetodoc.com/presentation_image/285dbb20d1be9b70051c2d926bd8ecb4/image-20.jpg)
NMC radiopharmaceuticals in 2017 [18 F]-FDG (fluorodeoxyglucose) § § § Breast cancer, Lymphomas Melanoma, Gastrointestinal cancer, Cervical & ovarian cancer, Lung, etc. [18 F]-Choline § Prostate cancer [68 Ga]-PSMA-11 § Prostate cancer § Other (? ? ? ) 20
![Plans for 2018 2019 18 FPSMA for early diagnostic of recurrent prostate Plans for 2018. – 2019. [18 F]-PSMA - for early diagnostic of recurrent prostate](https://slidetodoc.com/presentation_image/285dbb20d1be9b70051c2d926bd8ecb4/image-21.jpg)
Plans for 2018. – 2019. [18 F]-PSMA - for early diagnostic of recurrent prostate cancer. Hypoxia PET tracers – precise localization of cancer cells before radiation therapy (thyroid cancer, throat cancer etc. ): ü [18 F]-FMISO, [18 F]- FAZA or [18 F]-HX 4. . . to be decided upon availability of inlicensing. Diagnosis of Alzheimer: ü [18 F] Florbetaben (Neuraceq), Florbetapir (Amyvid ) or flutemetamol (Vizamyl ). . . to be decided upon availability of in-licensing. Theranostics approach: ü Using of 89 Zr isotope in combination with protein drugs – monoclonal antibodies, therapeutic peptides, enzymes, onco-viruses. 21

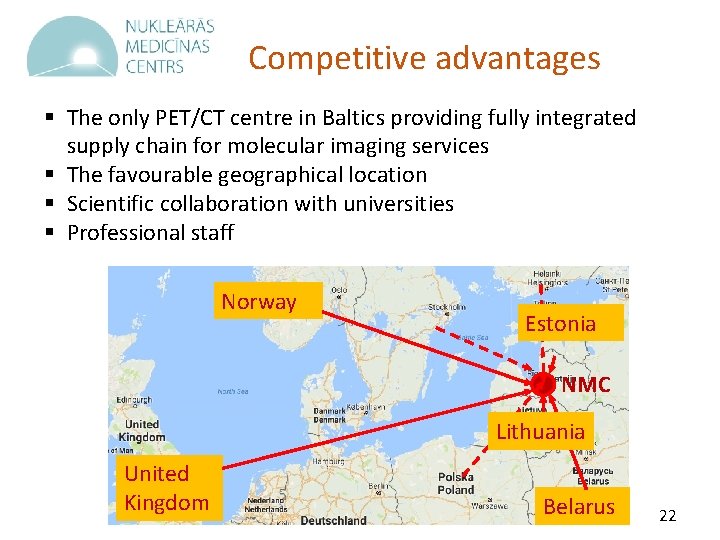
Competitive advantages § The only PET/CT centre in Baltics providing fully integrated supply chain for molecular imaging services § The favourable geographical location § Scientific collaboration with universities § Professional staff Norway Estonia NMC Lithuania United Kingdom Belarus 22

Welcome to NMC Nuclear Medicine Center Gardenes str. 13, Riga, Latvia E-mail: info@rnmc. lv WEB: www. rnmc. lv For PET/CT cancer diagnostics E-mail: info@rsunuclearmed. com Tel. : +371 27 078 822 Email: info@rsunuclearmed. com WEB: www. rsunuclearmed. com 23
 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Nmis nuclear medicine software
Nmis nuclear medicine software Measles artifact nuclear medicine
Measles artifact nuclear medicine Spatial resolution
Spatial resolution Mt sinai nuclear medicine
Mt sinai nuclear medicine Filtered back projection
Filtered back projection Diaphragm
Diaphragm Nuclear medicine information system
Nuclear medicine information system Nuclear medicine lectures
Nuclear medicine lectures Conclusion for case study
Conclusion for case study George armistead riga
George armistead riga Riga cripto
Riga cripto Riga acitivities
Riga acitivities Lettura buretta
Lettura buretta Alan turing
Alan turing Ritratto con la riga verde matisse
Ritratto con la riga verde matisse Luxexpress promo code
Luxexpress promo code Restourants in riga
Restourants in riga Costruzioni con riga e compasso
Costruzioni con riga e compasso Planetārijs rīgā
Planetārijs rīgā Stolica lotwa
Stolica lotwa Bomax locksmith
Bomax locksmith Janis rozentāls riga art school
Janis rozentāls riga art school