Rh Grouping PRACTICAL ASPECTS OF RH GROUPING Rh









- Slides: 19

Rh Grouping

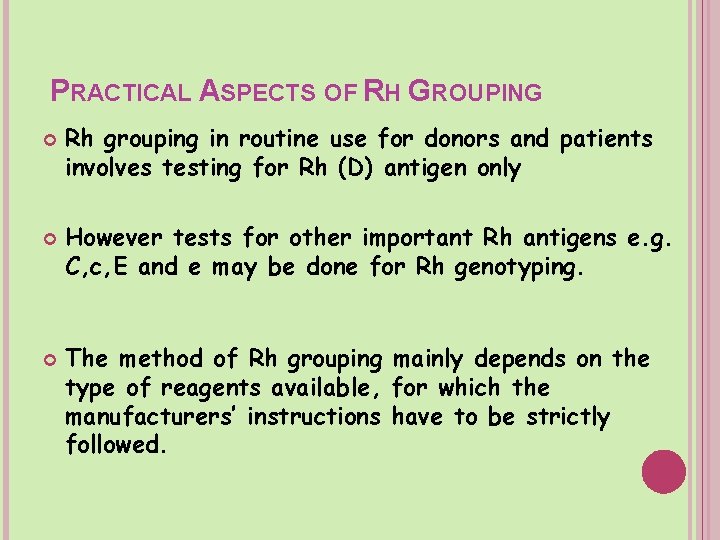
PRACTICAL ASPECTS OF RH GROUPING Rh grouping in routine use for donors and patients involves testing for Rh (D) antigen only However tests for other important Rh antigens e. g. C, c, E and e may be done for Rh genotyping. The method of Rh grouping mainly depends on the type of reagents available, for which the manufacturers’ instructions have to be strictly followed.

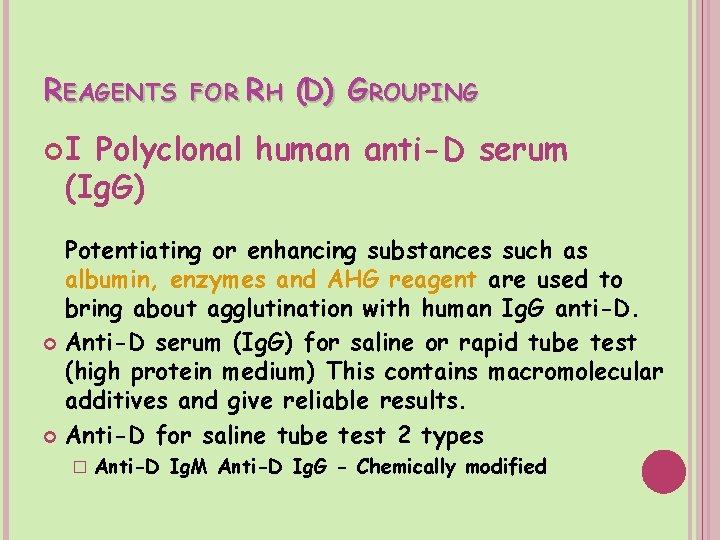
REAGENTS FOR RH (D) GROUPING I Polyclonal human anti-D serum (Ig. G) Potentiating or enhancing substances such as albumin, enzymes and AHG reagent are used to bring about agglutination with human Ig. G anti-D. Anti-D serum (Ig. G) for saline or rapid tube test (high protein medium) This contains macromolecular additives and give reliable results. Anti-D for saline tube test 2 types � Anti-D Ig. M Anti-D Ig. G - Chemically modified

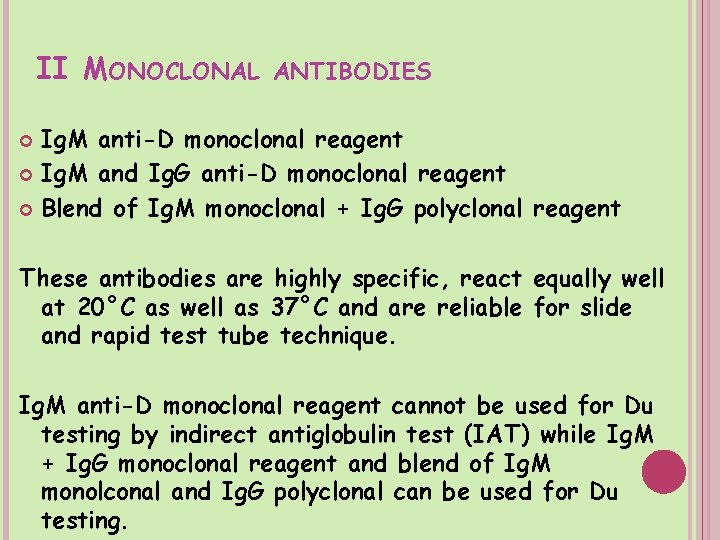
II MONOCLONAL ANTIBODIES Ig. M anti-D monoclonal reagent Ig. M and Ig. G anti-D monoclonal reagent Blend of Ig. M monoclonal + Ig. G polyclonal reagent These antibodies are highly specific, react equally well at 20°C as well as 37°C and are reliable for slide and rapid test tube technique. Ig. M anti-D monoclonal reagent cannot be used for Du testing by indirect antiglobulin test (IAT) while Ig. M + Ig. G monoclonal reagent and blend of Ig. M monolconal and Ig. G polyclonal can be used for Du testing.

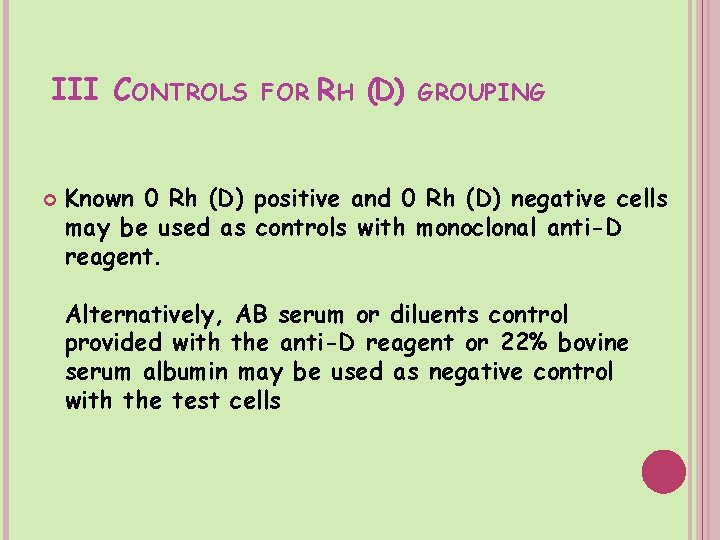
III CONTROLS FOR RH (D) GROUPING Known 0 Rh (D) positive and 0 Rh (D) negative cells may be used as controls with monoclonal anti-D reagent. Alternatively, AB serum or diluents control provided with the anti-D reagent or 22% bovine serum albumin may be used as negative control with the test cells

RH (D) GROUPING In most of the blood transfusion laboratories, Rh (D) grouping is performed along with the ABO grouping and same techniques as used for ABO grouping may also be employed for Rh typing

SLIDE TECHNIQUE This technique may be used in emergency Rh (D) typing if a centrifuge is not available. The slide test is not recommended for routine test as it may not pick up weak reactions, thus giving negative results.

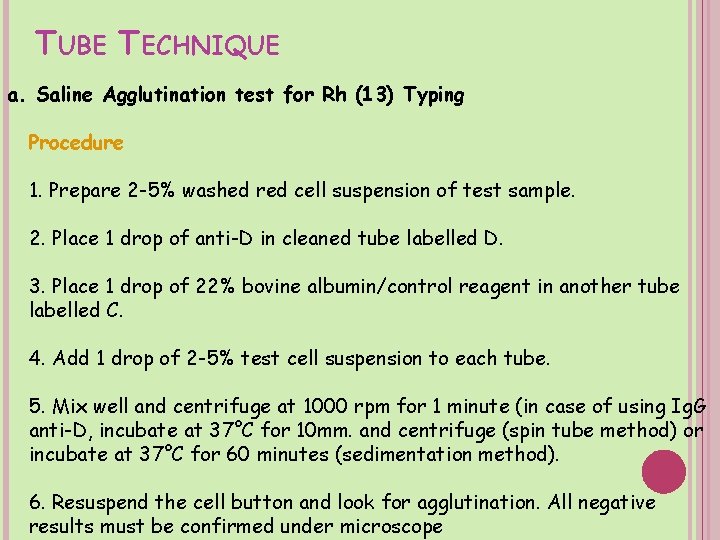
TUBE TECHNIQUE a. Saline Agglutination test for Rh (13) Typing Procedure 1. Prepare 2 -5% washed red cell suspension of test sample. 2. Place 1 drop of anti-D in cleaned tube labelled D. 3. Place 1 drop of 22% bovine albumin/control reagent in another tube labelled C. 4. Add 1 drop of 2 -5% test cell suspension to each tube. 5. Mix well and centrifuge at 1000 rpm for 1 minute (in case of using Ig. G anti-D, incubate at 37°C for 10 mm. and centrifuge (spin tube method) or incubate at 37°C for 60 minutes (sedimentation method). 6. Resuspend the cell button and look for agglutination. All negative results must be confirmed under microscope

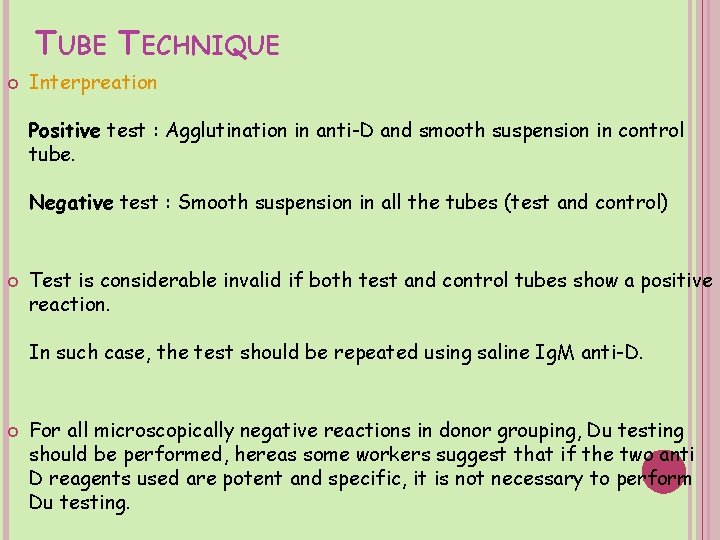
TUBE TECHNIQUE Interpreation Positive test : Agglutination in anti-D and smooth suspension in control tube. Negative test : Smooth suspension in all the tubes (test and control) Test is considerable invalid if both test and control tubes show a positive reaction. In such case, the test should be repeated using saline Ig. M anti-D. For all microscopically negative reactions in donor grouping, Du testing should be performed, hereas some workers suggest that if the two anti D reagents used are potent and specific, it is not necessary to perform Du testing.

ALBUMIN TECHNIQUE FOR RH (D) TYPING Principle Albumin increases the dielectric constant of the medium and thus reduces the zeta potential. Due to this effect, the electrical repulsion between the red blood cells is less and the cells agglutinate. Mostly 22% bovine albumin is used, as higher concentrations can cause rouleaux formation.

ENZYME AGGLUTINATION (D) TYPING TECHNIQUE FOR RH Proteolytic enzymes such as papain, trypsin, bromelain and ficin digest the cell membrane partially and expose Rh antigens to react with Ig. G antibodies. When the membrane is partially removed, it brings about a loss of negative electric charge on the red cell which is responsible for keeping the cells a set distance apart, hence small Ig. G molecules are able to span the gap between cells and bring about agglutination.

RH(D) GROUPING IN HAEMOLYTLC DISEASE THE NEWBORN OF In haemolytic disease of the newborn, the baby’s red cells may be coated with immunoglobulin and a saline reactive Rh antiserum is usually necessary for testing. When the cells are heavily coated with antibody, no free antigenic sites remain for reaction, resulting in a negative test. This is suspected when the infant’s cells show a positive direct antiglobulin test (DAT) and a negative test with anti-D reagent. In such instances it is recommended that the antibody should be eluted by gentle elution (heating at 45°C for 30 minutes) to expose the antigenic sites before testing.

RH DISCREPANCIES Rh –ve persons mistyped, & transfused with Rh +ve blood have 70% chance of becoming immunized False +ve reactions can be identified by testing an Rh control with the patient’s red cells each time an Rh typing is performed

CAUSES OF FALSE POSITIVE REACTIONS 1. 2. 3. Positive direct antiglobulin test Polagglutinable red cells Cold agglutinins or Rouleaux formation

1 - POSITIVE DIRECT ANTIGLOBULIN TEST The presence of Ab on patient’s red cells can cause a false +ve reaction with slide and tube anti-D High protein medium reduces zeta potential allowing red cells to move closer The cell bound Ab can cross link cells and cause agglutination

2 - POLAGGLUTINABLE RED CELLS Rh –ve red cells that are polyagglutinable due to T or Tn activation Agglutination occurs if anti-T or anti-Tn present in the anti-D reagent Most anti-D reagents do not contain these antibodies

3 - COLD AGGLUTININS OR ROULEAUX FORMATION Rh typing is performed using serum suspended red cells If individual’s serum contains cold agglutinin or abnormal protein, false positive reactions can occur

FALSE NEGATIVES False negatives are not readily identifiable, but can occur in the following instances: � The most common is the result of too heavy cell suspension due to too many cells for the amount of antibody in the antisera. � They may also rarely be caused by extremely strong positive DAT. In this case a the patient's D antigen sites are coated in vivo and there are no sites left for commercial anti-D to attach to. This can be fixed by heating cells gently to elute off antibody without damaging cells, then re-test.

RESOLVING RH PROBLEMS Erroneous Results in Rh Grouping 1. Perform clerical checks for validity of labels and requisition forms. 2. Obtain a fresh blood sample of patient. 3. Check patient rçcords for history, diagnosis, pregnancy, medication and previous transfusion. 4. Check equipment and reagents for proper quality control. 5. Check the antisera and controls. 6. Perform alternative procedures such as washing of red cells with warm saline, enzyme treatment of red cells, absorption / elution studies.
 Practical aspects meaning
Practical aspects meaning 4 aspects of research
4 aspects of research Aspects of attitude
Aspects of attitude Features of romanticism
Features of romanticism 3 aspects of tawheed
3 aspects of tawheed Psychosocial aspects of living with diabetes
Psychosocial aspects of living with diabetes Value oriented retail strategy
Value oriented retail strategy Aspects of responsibility
Aspects of responsibility All aspects of software production
All aspects of software production Legal aspects of doing business in canada
Legal aspects of doing business in canada Literary elements of drama
Literary elements of drama Crucial aspects of preparing digital audio files
Crucial aspects of preparing digital audio files Ethical and legal issues in community health nursing
Ethical and legal issues in community health nursing Perfect aspect
Perfect aspect Aristotelian tragic hero
Aristotelian tragic hero Aspects of american dream
Aspects of american dream The recurring aspects of designs are called design
The recurring aspects of designs are called design Involves managing all aspects of a customer relationship
Involves managing all aspects of a customer relationship Projection defense mechanism
Projection defense mechanism Irving isd v tatro
Irving isd v tatro