REWARDS Premier TLX Percutaneous Coronary Intervention with Promus












- Slides: 27

REWARDS Premier TLX: Percutaneous Coronary Intervention with Promus Premier vs. Xience V and Taxus Liberte in Contemporary US Practice Michael A. Gaglia, Jr. , MD, MSc, FACC, FSCAI On behalf of the REWARDS Premier TLX Investigators Medstar Cardiovascular Research Network Medstar Heart and Vascular Institute

Michael A. Gaglia, Jr. The Medstar Health Research Institute received an unrestricted research grant from Boston Scientific to fund this study.

Promus Element vs. Xience V: PLATINUM Target Lesion Failure (%) Per Protocol Intention-to-Treat Co. Cr-EES (N=747) 10 Co. Cr-EES (N=762) 10 Pt. Cr-EES (N=756) Pt. Cr-EES (N=768) 8 8 HR [95% CI] = 1. 17 [0. 66, 2. 09] P = 0. 59 6 4 HR [95% CI] = 1. 12 [0. 64, 1. 95] P = 0. 70 6 3. 4% 3. 0% 2 0 4 3. 5% 3. 2% 2 0 0 3 9 12 0 3 Months No. at risk Co. Cr EES 747 Pt. Cr 756 EES 6 6 9 12 Months 735 731 723 707 762 747 743 735 718 745 740 734 719 768 756 751 745 730 Stone GW et al. JACC 2011; 57: 1700

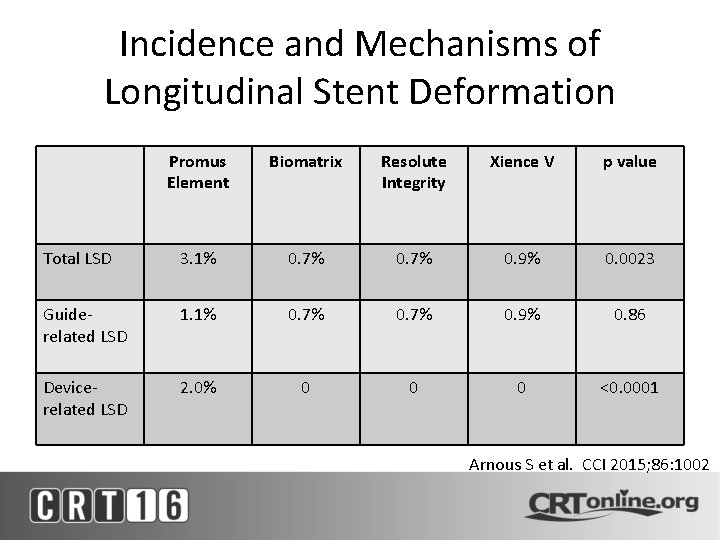
Incidence and Mechanisms of Longitudinal Stent Deformation Promus Element Biomatrix Resolute Integrity Xience V p value Total LSD 3. 1% 0. 7% 0. 9% 0. 0023 Guiderelated LSD 1. 1% 0. 7% 0. 9% 0. 86 Devicerelated LSD 2. 0% 0 0 0 <0. 0001 Arnous S et al. CCI 2015; 86: 1002

Promus Element vs. Promus Premier Ormiston JA et al. Circ Cardiovasc Int 2014; 7: 62

Longitudinal Stent Deformation: Promus Element vs. Promus Premier Ormiston JA et al. Circ Cardiovasc Int 2014; 7: 62

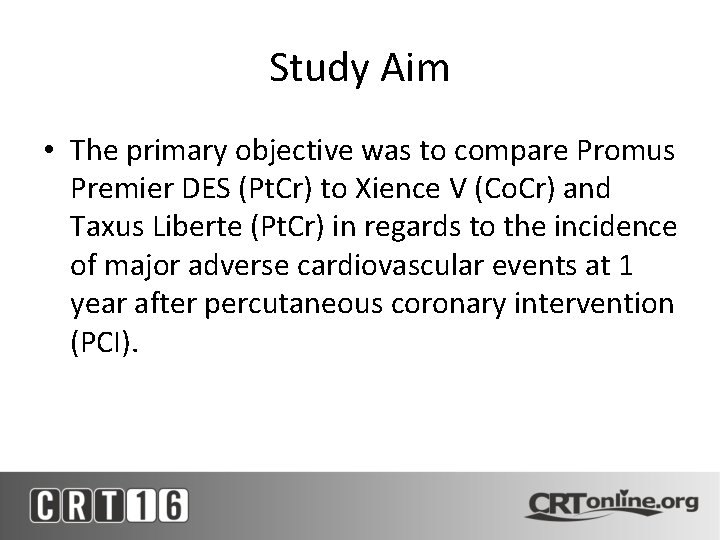
Study Aim • The primary objective was to compare Promus Premier DES (Pt. Cr) to Xience V (Co. Cr) and Taxus Liberte (Pt. Cr) in regards to the incidence of major adverse cardiovascular events at 1 year after percutaneous coronary intervention (PCI).

REWARDS Premier TLX Sites Site Primary Investigator Total Enrolled Washington Hospital Center, Washington, DC Ron Waksman, MD 101 Medical University of South Carolina, Charleston, SC Daniel Steinberg, MD 38 Medstar Union Memorial Hospital, Baltimore, MD John Wang, MD 49 Grand Strand Regional Medical Center, Myrtle Beach, SC Randy Goodroe, MD 269 Columbia University Medical Center, New York, NY Tamim Nazif, MD 71 Prairie Education and Research Cooperative, Springfield, IL Greg Mishkel, MD 167 Sinai Hospital, Baltimore, MD Ali Tabrizchi, DO 86 Geisinger Medical Center, Danville, PA Thomas Scott, DO 41 West Virginia University Heart Institute, Morgantown, WV Wassim Gharib, MD 87 Charlotte Heart Research Group, Port Charlotte, FL Mario Lopez, MD 41

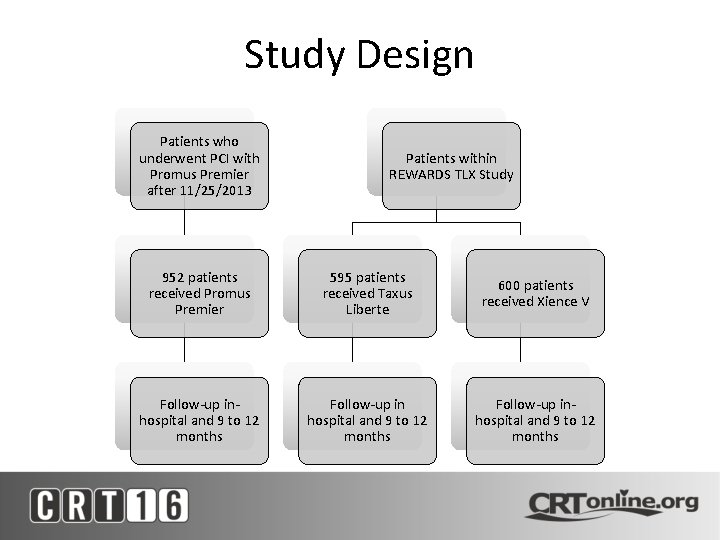
Study Design Patients who underwent PCI with Promus Premier after 11/25/2013 Patients within REWARDS TLX Study 952 patients received Promus Premier 595 patients received Taxus Liberte 600 patients received Xience V Follow-up inhospital and 9 to 12 months Follow-up inhospital and 9 to 12 months

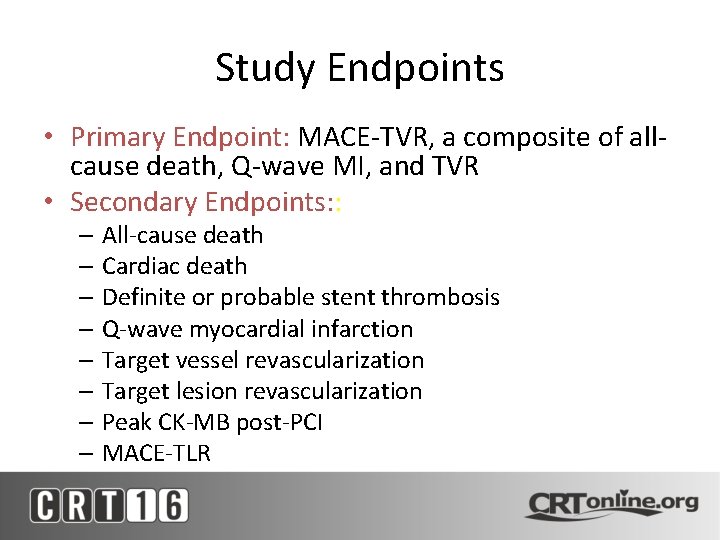
Study Endpoints • Primary Endpoint: MACE-TVR, a composite of allcause death, Q-wave MI, and TVR • Secondary Endpoints: : – All-cause death – Cardiac death – Definite or probable stent thrombosis – Q-wave myocardial infarction – Target vessel revascularization – Target lesion revascularization – Peak CK-MB post-PCI – MACE-TLR

Inclusion/Exclusion Criteria: Premier Cohort • Inclusion Criteria – Subject > 18 years of age – Underwent PCI with Promus Premier (alone) DES • Exclusion Criteria – Underwent PCI with a non-Promus Premier DES during the same index procedure – Received a combination of Promus Premier and Taxus Liberte OR Xience V during the index procedure – Patients not taking, or unable to take, dual antiplatelet therapy (aspirin plus clopidogrel, prasugrel, or ticagrelor).

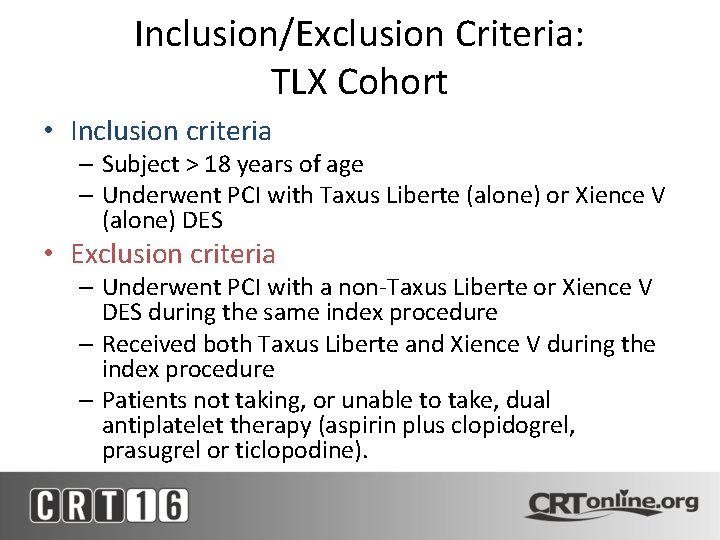
Inclusion/Exclusion Criteria: TLX Cohort • Inclusion criteria – Subject > 18 years of age – Underwent PCI with Taxus Liberte (alone) or Xience V (alone) DES • Exclusion criteria – Underwent PCI with a non-Taxus Liberte or Xience V DES during the same index procedure – Received both Taxus Liberte and Xience V during the index procedure – Patients not taking, or unable to take, dual antiplatelet therapy (aspirin plus clopidogrel, prasugrel or ticlopodine).

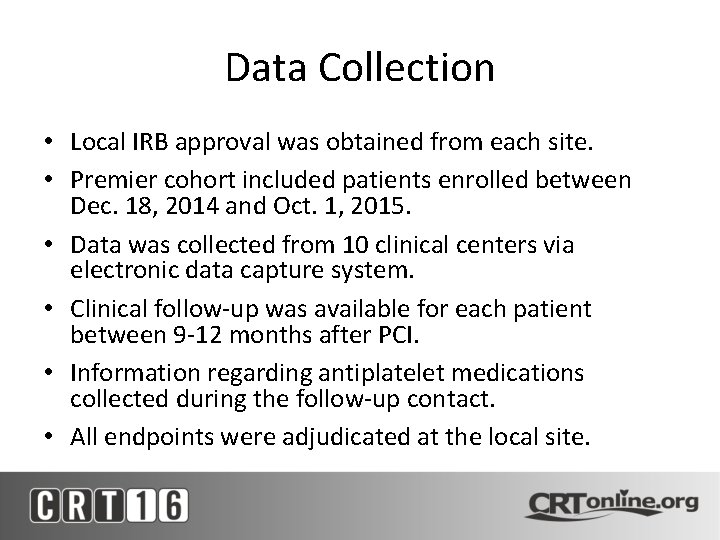
Data Collection • Local IRB approval was obtained from each site. • Premier cohort included patients enrolled between Dec. 18, 2014 and Oct. 1, 2015. • Data was collected from 10 clinical centers via electronic data capture system. • Clinical follow-up was available for each patient between 9 -12 months after PCI. • Information regarding antiplatelet medications collected during the follow-up contact. • All endpoints were adjudicated at the local site.

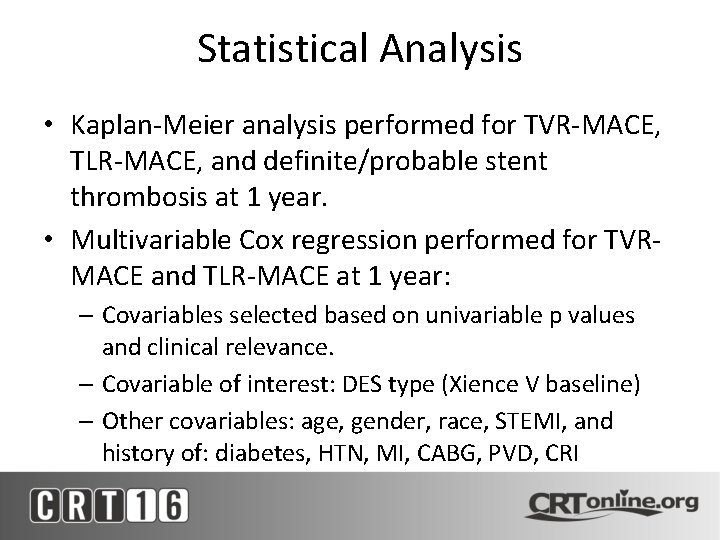
Statistical Analysis • Kaplan-Meier analysis performed for TVR-MACE, TLR-MACE, and definite/probable stent thrombosis at 1 year. • Multivariable Cox regression performed for TVRMACE and TLR-MACE at 1 year: – Covariables selected based on univariable p values and clinical relevance. – Covariable of interest: DES type (Xience V baseline) – Other covariables: age, gender, race, STEMI, and history of: diabetes, HTN, MI, CABG, PVD, CRI

Baseline Characteristics Promus Premier Taxus Liberte Xience V p value Age 65. 3 ± 11. 1 64. 6 ± 11. 3 63. 2 ± 11. 2 <0. 001 Male 69. 1% 69. 9% 69. 3% 0. 95 Caucasian 89. 7% 92. 5% 75. 3% <0. 001 Hispanic 1. 8% 3. 0% 1. 2% 0. 15 Current smoking 44. 6% 30. 5% 30. 1% <0. 001 Diabetes 36. 9% 33. 4% 35. 2% 0. 39 IDDM 14. 1% 8. 2% 9. 7% <0. 001 Previous MI 25. 0% 23. 9% 23. 0% 0. 66 CABG 22. 4% 21. 8% 20. 8% 0. 77 Hypertension 85. 3% 75. 0% 84. 3% <0. 001 Renal Insufficiency 8. 8% 5. 5% 6. 7% 0. 04 PVD 11. 6% 9. 2% 11. 8% 0. 27

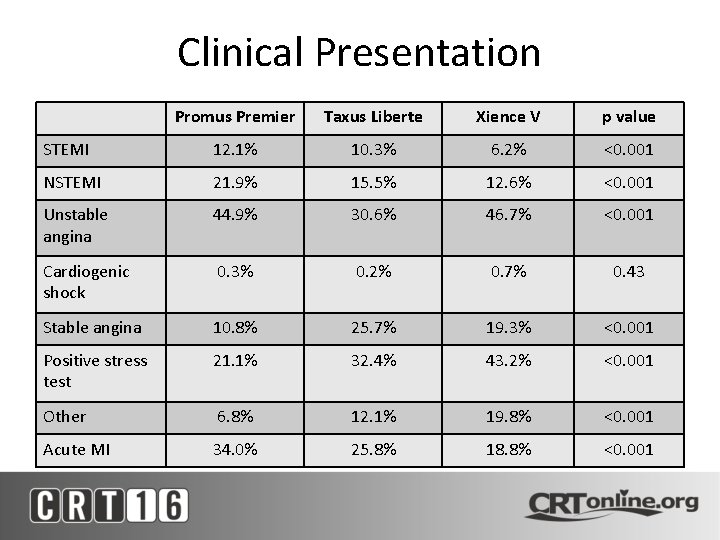
Clinical Presentation Promus Premier Taxus Liberte Xience V p value STEMI 12. 1% 10. 3% 6. 2% <0. 001 NSTEMI 21. 9% 15. 5% 12. 6% <0. 001 Unstable angina 44. 9% 30. 6% 46. 7% <0. 001 Cardiogenic shock 0. 3% 0. 2% 0. 7% 0. 43 Stable angina 10. 8% 25. 7% 19. 3% <0. 001 Positive stress test 21. 1% 32. 4% 43. 2% <0. 001 Other 6. 8% 12. 1% 19. 8% <0. 001 Acute MI 34. 0% 25. 8% 18. 8% <0. 001

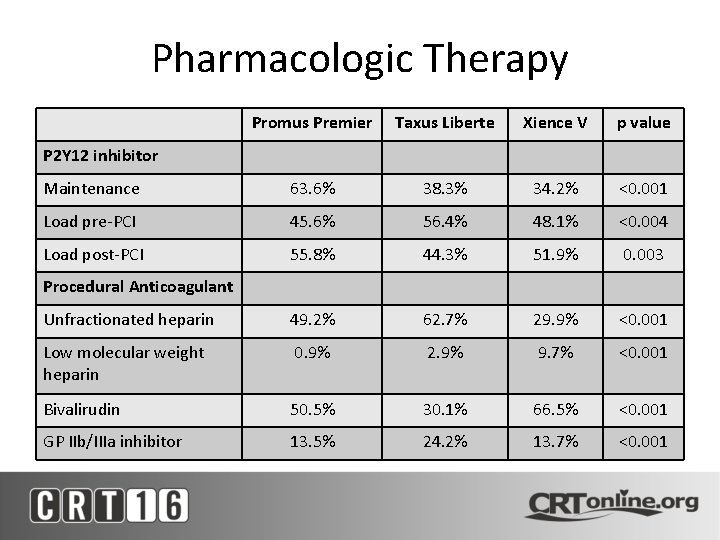
Pharmacologic Therapy Promus Premier Taxus Liberte Xience V p value Maintenance 63. 6% 38. 3% 34. 2% <0. 001 Load pre-PCI 45. 6% 56. 4% 48. 1% <0. 004 Load post-PCI 55. 8% 44. 3% 51. 9% 0. 003 Unfractionated heparin 49. 2% 62. 7% 29. 9% <0. 001 Low molecular weight heparin 0. 9% 2. 9% 9. 7% <0. 001 Bivalirudin 50. 5% 30. 1% 66. 5% <0. 001 GP IIb/IIIa inhibitor 13. 5% 24. 2% 13. 7% <0. 001 P 2 Y 12 inhibitor Procedural Anticoagulant

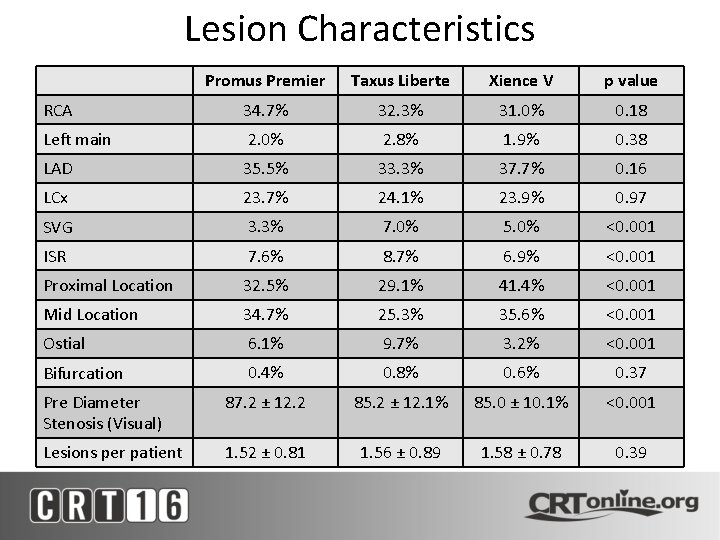
Lesion Characteristics Promus Premier Taxus Liberte Xience V p value RCA 34. 7% 32. 3% 31. 0% 0. 18 Left main 2. 0% 2. 8% 1. 9% 0. 38 LAD 35. 5% 33. 3% 37. 7% 0. 16 LCx 23. 7% 24. 1% 23. 9% 0. 97 SVG 3. 3% 7. 0% 5. 0% <0. 001 ISR 7. 6% 8. 7% 6. 9% <0. 001 Proximal Location 32. 5% 29. 1% 41. 4% <0. 001 Mid Location 34. 7% 25. 3% 35. 6% <0. 001 Ostial 6. 1% 9. 7% 3. 2% <0. 001 Bifurcation 0. 4% 0. 8% 0. 6% 0. 37 Pre Diameter Stenosis (Visual) 87. 2 ± 12. 2 85. 2 ± 12. 1% 85. 0 ± 10. 1% <0. 001 Lesions per patient 1. 52 ± 0. 81 1. 56 ± 0. 89 1. 58 ± 0. 78 0. 39

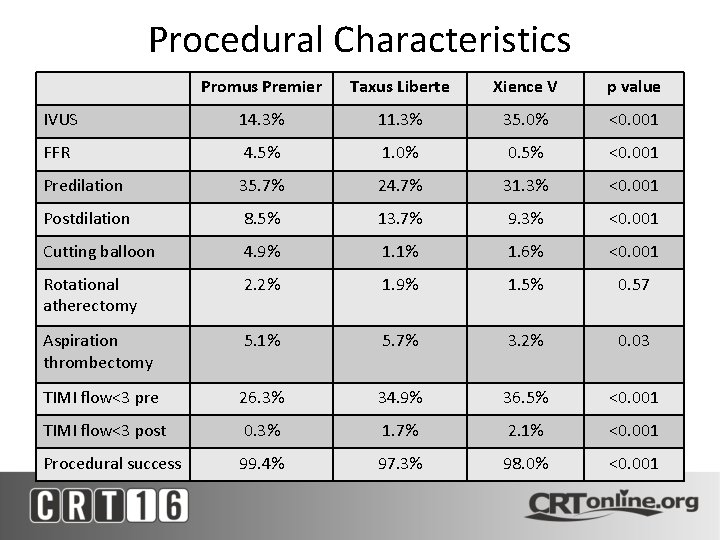
Procedural Characteristics Promus Premier Taxus Liberte Xience V p value IVUS 14. 3% 11. 3% 35. 0% <0. 001 FFR 4. 5% 1. 0% 0. 5% <0. 001 Predilation 35. 7% 24. 7% 31. 3% <0. 001 Postdilation 8. 5% 13. 7% 9. 3% <0. 001 Cutting balloon 4. 9% 1. 1% 1. 6% <0. 001 Rotational atherectomy 2. 2% 1. 9% 1. 5% 0. 57 Aspiration thrombectomy 5. 1% 5. 7% 3. 2% 0. 03 TIMI flow<3 pre 26. 3% 34. 9% 36. 5% <0. 001 TIMI flow<3 post 0. 3% 1. 7% 2. 1% <0. 001 Procedural success 99. 4% 97. 3% 98. 0% <0. 001

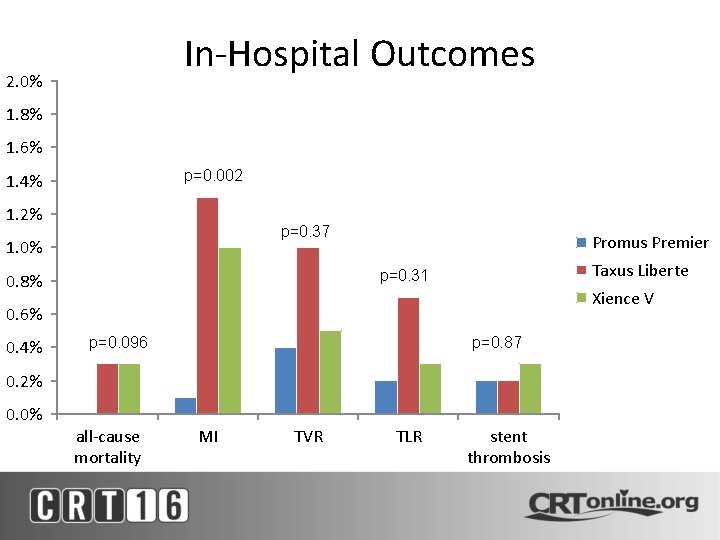
In-Hospital Outcomes 2. 0% 1. 8% 1. 6% p=0. 002 1. 4% 1. 2% p=0. 37 1. 0% Promus Premier Taxus Liberte p=0. 31 0. 8% Xience V 0. 6% 0. 4% p=0. 096 p=0. 87 0. 2% 0. 0% all-cause mortality MI TVR TLR stent thrombosis

Outcomes at Follow-Up 12. 0% p=0. 001 10. 0% p<0. 001 8. 0% Promus Premier 6. 0% Xience V 4. 0% 2. 0% 0. 0% Taxus Liberte p=0. 15 p=0. 32 p=0. 044 p=0. 012 death MI TVR TLR ST TVR-MACE

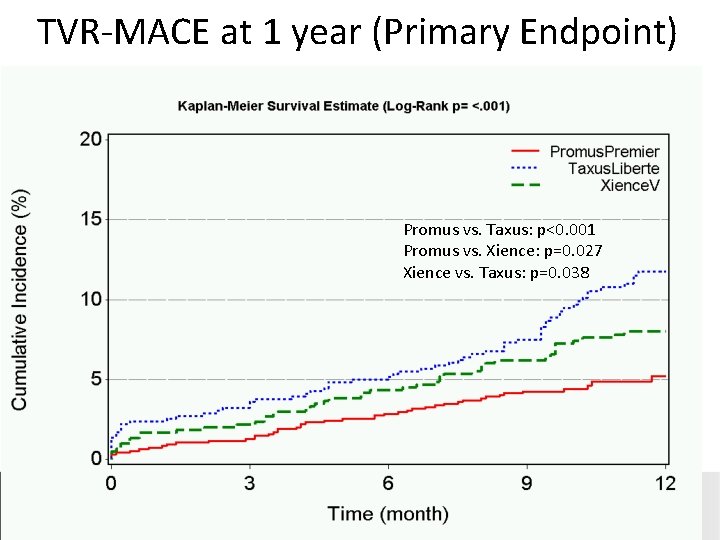
TVR-MACE at 1 year (Primary Endpoint) Promus vs. Taxus: p<0. 001 Promus vs. Xience: p=0. 027 Xience vs. Taxus: p=0. 038

TLR-MACE at 1 Year Promus vs. Taxus: p=0. 003 Promus vs. Xience: p=0. 08 Xience vs. Taxus: p=0. 19

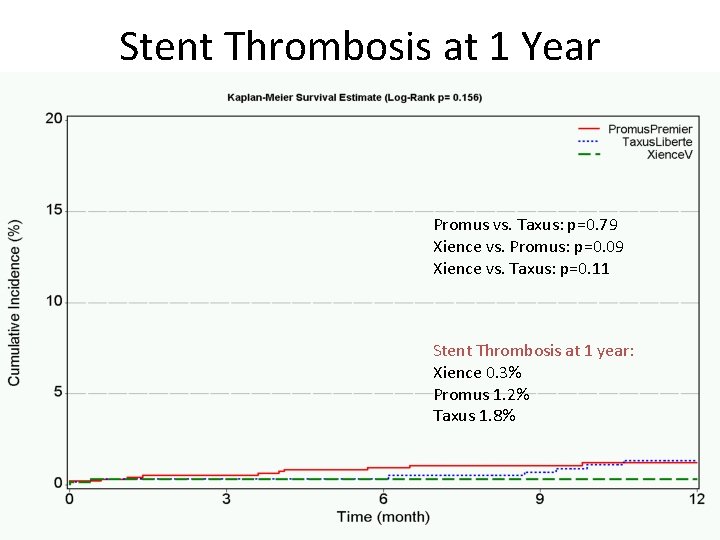
Stent Thrombosis at 1 Year Promus vs. Taxus: p=0. 79 Xience vs. Promus: p=0. 09 Xience vs. Taxus: p=0. 11 Stent Thrombosis at 1 year: Xience 0. 3% Promus 1. 2% Taxus 1. 8%

Cox Regression for TVR-MACE Hazard Ratio 95% Confidence Interval p value Promus Premier 0. 63 0. 41 -0. 96 0. 03 Taxus Liberte 1. 47 0. 99 -2. 17 0. 054 Age (per 10 y) 0. 86 0. 74 -1. 01 0. 059 Female 1. 18 0. 84 -1. 67 0. 34 African-American 0. 95 0. 56 -1. 60 0. 84 STEMI 1. 19 0. 69 -2. 07 0. 53 Diabetes 1. 19 0. 85 -1. 67 0. 31 CABG 1. 60 1. 11 -2. 31 0. 01 PVD 1. 47 0. 94 -2. 32 0. 09 CRI 1. 42 0. 83 -2. 43 0. 20 Previous MI 1. 42 1. 00 -2. 01 0. 047 Hypertension 1. 30 0. 80 -2. 11 0. 29

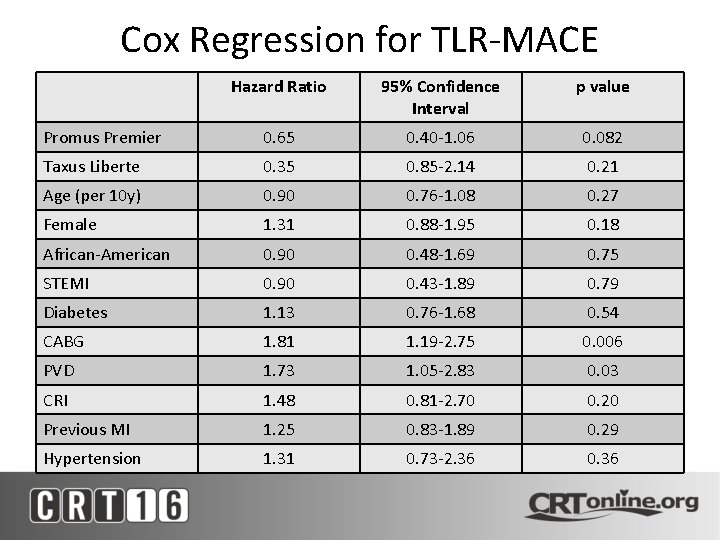
Cox Regression for TLR-MACE Hazard Ratio 95% Confidence Interval p value Promus Premier 0. 65 0. 40 -1. 06 0. 082 Taxus Liberte 0. 35 0. 85 -2. 14 0. 21 Age (per 10 y) 0. 90 0. 76 -1. 08 0. 27 Female 1. 31 0. 88 -1. 95 0. 18 African-American 0. 90 0. 48 -1. 69 0. 75 STEMI 0. 90 0. 43 -1. 89 0. 79 Diabetes 1. 13 0. 76 -1. 68 0. 54 CABG 1. 81 1. 19 -2. 75 0. 006 PVD 1. 73 1. 05 -2. 83 0. 03 CRI 1. 48 0. 81 -2. 70 0. 20 Previous MI 1. 25 0. 83 -1. 89 0. 29 Hypertension 1. 31 0. 73 -2. 36 0. 36

Summary • This real-world registry demonstrates the safety and efficacy of the Promus Premier DES in a wide variety of patients and lesions. – Connectors to attenuate LSD did not appear to decrease procedural success. • Stent thrombosis at 1 year still reasonably low (1. 2%) despite one-third acute MI patients in Premier cohort. • Promus Premier compared favorably to Xience V for both in-hospital and 1 year outcomes. • TVR-MACE lower with Promus Premier compared to both Xience V and Taxus Liberte at 1 year. – Promus Premier independently associated with lower TVRMACE after adjustment for confounders.
 Methode premier entré premier sortie
Methode premier entré premier sortie Percutaneous image-guided lumbar decompression
Percutaneous image-guided lumbar decompression Percutaneous transhepatic cholangiography and drainage
Percutaneous transhepatic cholangiography and drainage Percutaneous balloon pericardiotomy
Percutaneous balloon pericardiotomy Percutaneous nephrostomy
Percutaneous nephrostomy Choledocholithiasis
Choledocholithiasis Percutaneous umbilical blood sampling
Percutaneous umbilical blood sampling Coronary blood flow
Coronary blood flow Anterior cardiac veins of the right ventricle
Anterior cardiac veins of the right ventricle Heart wall
Heart wall Vein
Vein Heart disease
Heart disease Coronary circulation of heart
Coronary circulation of heart Frog heart
Frog heart Coronary groove of heart
Coronary groove of heart Right and left aortic sinus
Right and left aortic sinus Coronary angiography equipment
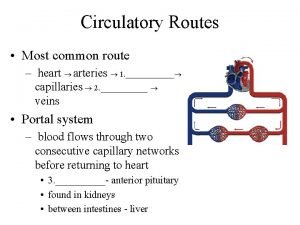
Coronary angiography equipment Coronary circulatory routes
Coronary circulatory routes Global registry of acute coronary events
Global registry of acute coronary events Coronary personality
Coronary personality Papyrus stent graft
Papyrus stent graft Function of coronary artery
Function of coronary artery Acute coronary syndrome
Acute coronary syndrome Coronary artery disease
Coronary artery disease Dipyrimadole
Dipyrimadole Coronary calcium score guidelines
Coronary calcium score guidelines Coronary artery disease pathophysiology
Coronary artery disease pathophysiology Blood vessels crash course
Blood vessels crash course