Revision of the gasphase acidity scale below 300
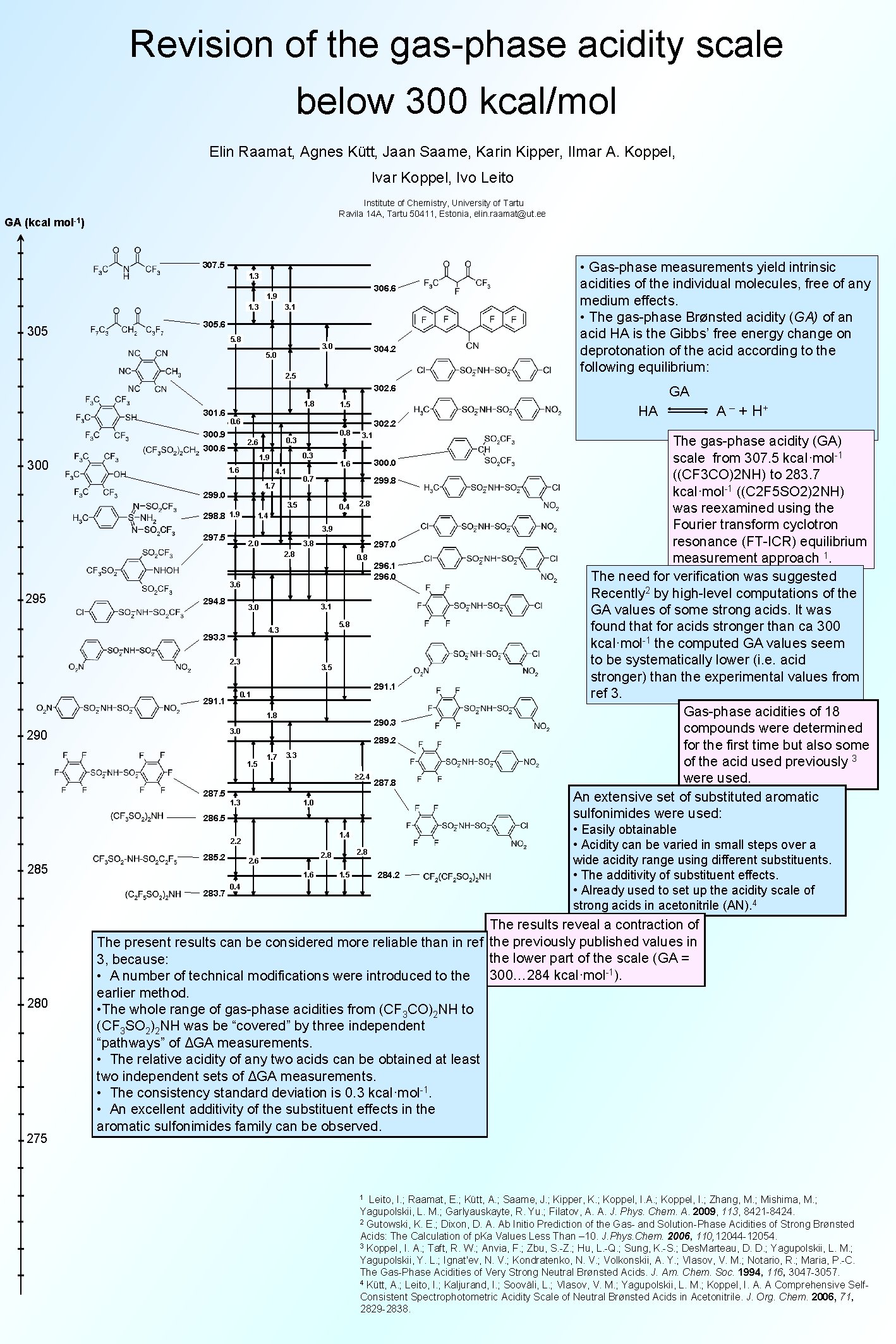
Revision of the gas-phase acidity scale below 300 kcal/mol Elin Raamat, Agnes Kütt, Jaan Saame, Karin Kipper, Ilmar A. Koppel, Ivar Koppel, Ivo Leito Institute of Chemistry, University of Tartu Ravila 14 A, Tartu 50411, Estonia, elin. raamat@ut. ee GA (kcal mol-1) 307. 5 1. 3 306. 6 1. 9 1. 3 305 3. 1 305. 6 5. 8 3. 0 304. 2 5. 0 2. 5 • Gas-phase measurements yield intrinsic acidities of the individual molecules, free of any medium effects. • The gas-phase Brønsted acidity (GA) of an acid HA is the Gibbs’ free energy change on deprotonation of the acid according to the following equilibrium: 302. 6 1. 8 301. 6 0. 6 300. 9 302. 2 0. 3 1. 9 1. 6 4. 1 300. 0 1. 6 299. 8 3. 5 298. 8 1. 9 3. 1 0. 7 1. 7 299. 0 0. 8 0. 3 2. 6 300 1. 5 0. 4 2. 8 1. 4 3. 9 297. 5 2. 0 3. 8 297. 0 2. 8 0. 8 3. 6 295 294. 8 3. 1 3. 0 5. 8 4. 3 293. 3 291. 1 3. 5 291. 1 0. 1 1. 8 290. 3 3. 0 296. 1 296. 0 289. 2 1. 5 1. 7 3. 3 ≥ 2. 4 287. 8 287. 5 1. 3 1. 0 286. 5 1. 4 2. 2 285. 2 280 275 2. 6 1. 6 283. 7 2. 8 284. 2 1. 5 0. 4 GA HA A – + H+ The gas-phase acidity (GA) scale from 307. 5 kcal·mol-1 ((CF 3 CO)2 NH) to 283. 7 kcal·mol-1 ((C 2 F 5 SO 2)2 NH) was reexamined using the Fourier transform cyclotron resonance (FT-ICR) equilibrium measurement approach 1. The need for verification was suggested Recently 2 by high-level computations of the GA values of some strong acids. It was found that for acids stronger than ca 300 kcal·mol-1 the computed GA values seem to be systematically lower (i. e. acid stronger) than the experimental values from ref 3. Gas-phase acidities of 18 compounds were determined for the first time but also some of the acid used previously 3 were used. An extensive set of substituted aromatic sulfonimides were used: • Easily obtainable • Acidity can be varied in small steps over a wide acidity range using different substituents. • The additivity of substituent effects. • Already used to set up the acidity scale of strong acids in acetonitrile (AN). 4 The results reveal a contraction of The present results can be considered more reliable than in ref the previously published values in the lower part of the scale (GA = 3, because: • A number of technical modifications were introduced to the 300… 284 kcal·mol-1). earlier method. • The whole range of gas-phase acidities from (CF 3 CO)2 NH to (CF 3 SO 2)2 NH was be “covered” by three independent “pathways” of ΔGA measurements. • The relative acidity of any two acids can be obtained at least two independent sets of ΔGA measurements. • The consistency standard deviation is 0. 3 kcal·mol-1. • An excellent additivity of the substituent effects in the aromatic sulfonimides family can be observed. Leito, I. ; Raamat, E. ; Kütt, A. ; Saame, J. ; Kipper, K. ; Koppel, I. A. ; Koppel, I. ; Zhang, M. ; Mishima, M. ; Yagupolskii, L. M. ; Garlyauskayte, R. Yu. ; Filatov, A. A. J. Phys. Chem. A. 2009, 113, 8421 -8424. 2 Gutowski, K. E. ; Dixon, D. A. Ab Initio Prediction of the Gas- and Solution-Phase Acidities of Strong Brønsted Acids: The Calculation of p. Ka Values Less Than – 10. J. Phys. Chem. 2006, 110, 12044 -12054. 3 Koppe. I, I. A. ; Taft, R. W. ; Anvia, F. ; Zbu, S. -Z. ; Hu, L. -Q. ; Sung, K. -S. ; Des. Marteau, D. D. ; Yagupolskii, L. M. ; Yagupolskii, Y. L. ; Ignat'ev, N. V. ; Kondratenko, N. V. ; Volkonskii, A. Y. ; Vlasov, V. M. ; Notario, R. ; Maria, P. -C. The Gas-Phase Acidities of Very Strong Neutral Brønsted Acids. J. Am. Chem. Soc. 1994, 116, 3047 -3057. 4 Kütt, A. ; Leito, I. ; Kaljurand, I. ; Sooväli, L. ; Vlasov, V. M. ; Yagupolskii, L. M. ; Koppel, I. A. A Comprehensive Self. Consistent Spectrophotometric Acidity Scale of Neutral Brønsted Acids in Acetonitrile. J. Org. Chem. 2006, 71, 2829 -2838. 1
- Slides: 1