Revision Lesson Draw the table below in your

Revision Lesson
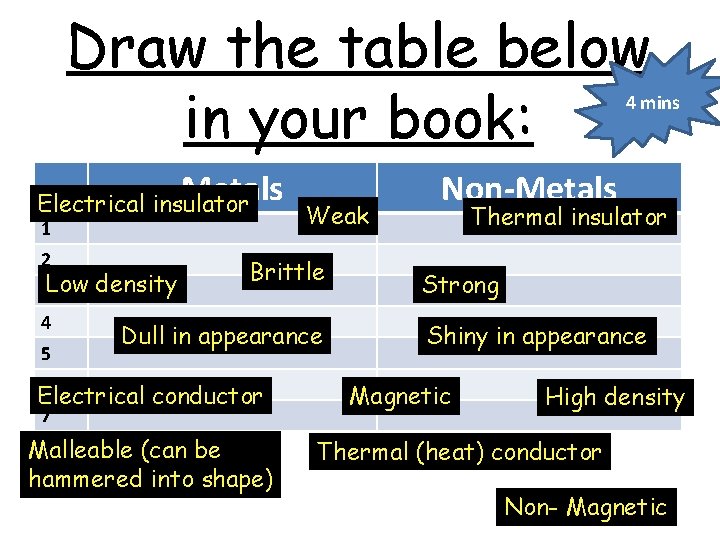
Draw the table below in your book: 4 mins Metals Electrical insulator 1 2 3 Low 4 5 density Weak Brittle Dull in appearance 6 Electrical conductor 7 Malleable (can be hammered into shape) Non-Metals Thermal insulator Strong Shiny in appearance Magnetic High density Thermal (heat) conductor Non- Magnetic
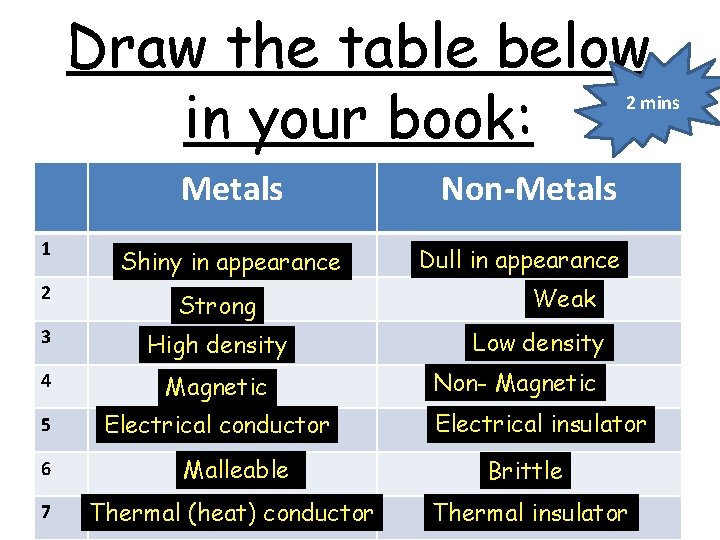
Draw the table below in your book: 2 mins Metals 1 Shiny in appearance 2 Strong 3 High density 4 Magnetic 5 Electrical conductor Non-Metals Dull in appearance Weak Low density Non- Magnetic Electrical insulator 6 Malleable Brittle 7 Thermal (heat) conductor Thermal insulator
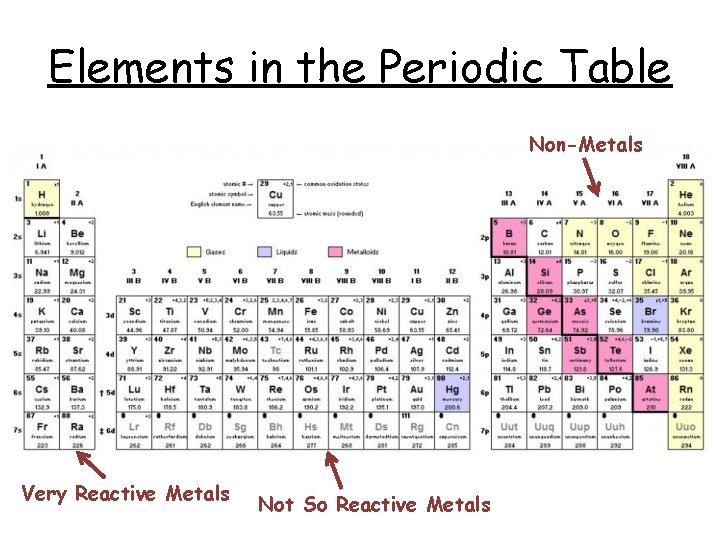
Elements in the Periodic Table Non-Metals Very Reactive Metals Not So Reactive Metals
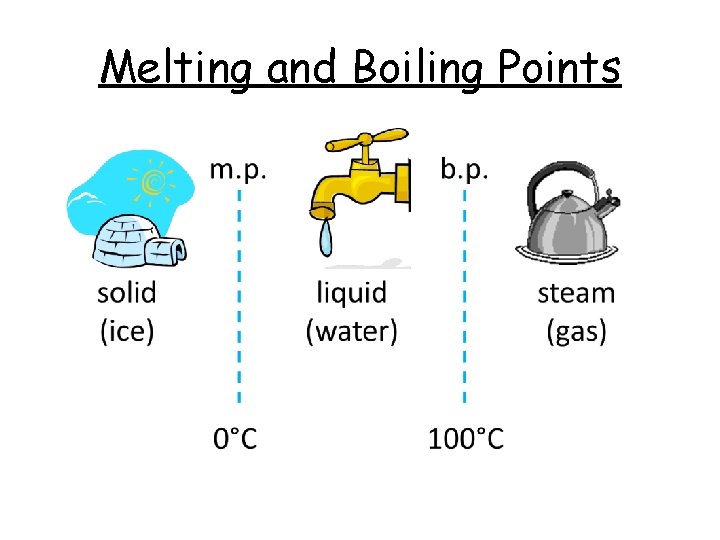
Melting and Boiling Points
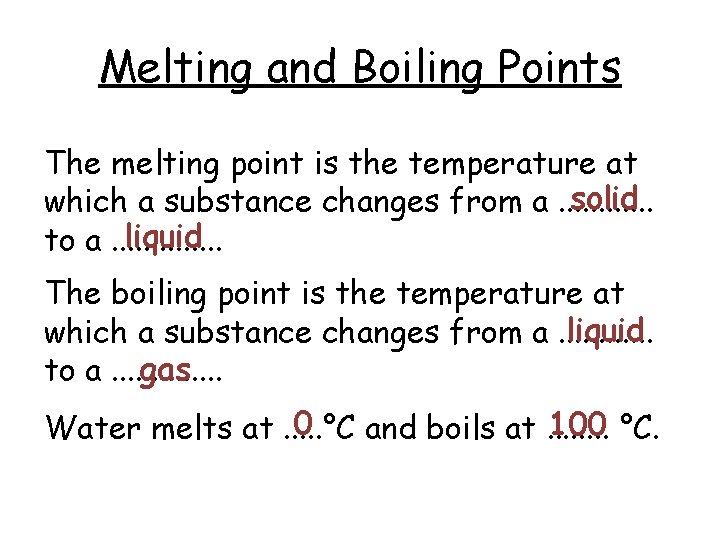
Melting and Boiling Points The melting point is the temperature at solid which a substance changes from a. . . liquid to a. . . The boiling point is the temperature at liquid which a substance changes from a. . . gas to a. . . 0 100 °C. Water melts at. . . °C and boils at. . . .
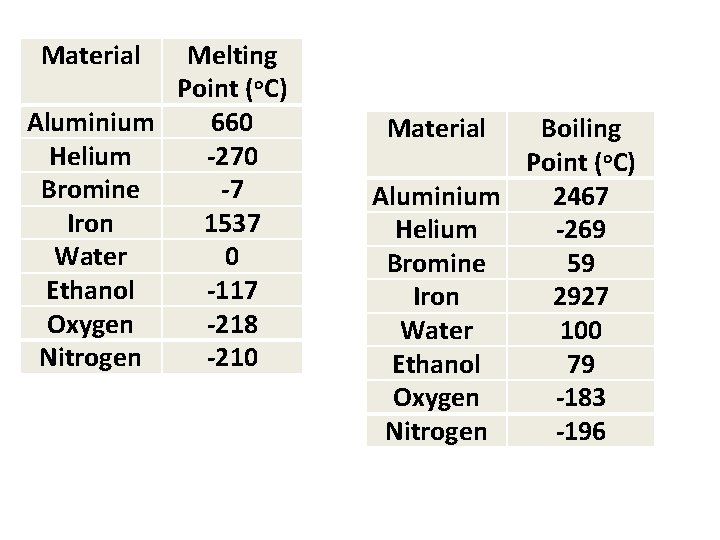
Material Melting Point (o. C) Aluminium 660 Helium -270 Bromine -7 Iron 1537 Water 0 Ethanol -117 Oxygen -218 Nitrogen -210 Material Boiling Point (o. C) Aluminium 2467 Helium -269 Bromine 59 Iron 2927 Water 100 Ethanol 79 Oxygen -183 Nitrogen -196

Separation Techniques A Mixture is formed from different substances that are not chemically joined together. Some mixtures are insoluble eg Sand Water Some mixtures are soluble eg Salt and Water
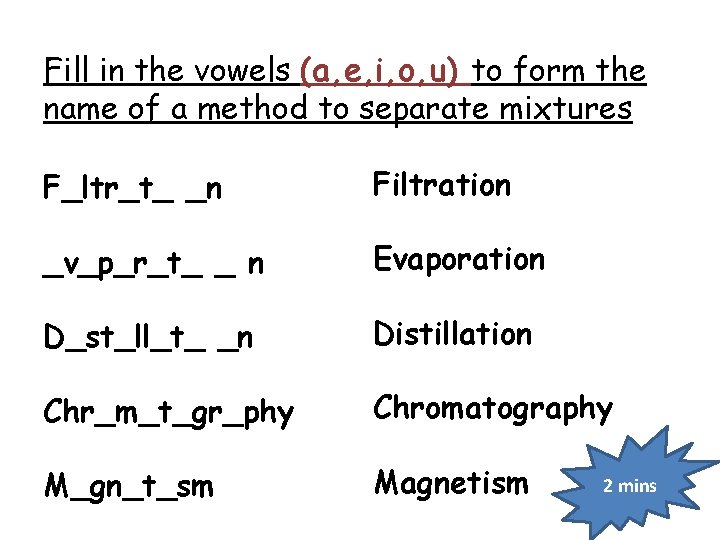
Fill in the vowels (a, e, i, o, u) to form the name of a method to separate mixtures F_ltr_t_ _n Filtration _v_p_r_t_ _ n Evaporation D_st_ll_t_ _n Distillation Chr_m_t_gr_phy Chromatography M_gn_t_sm Magnetism 2 mins
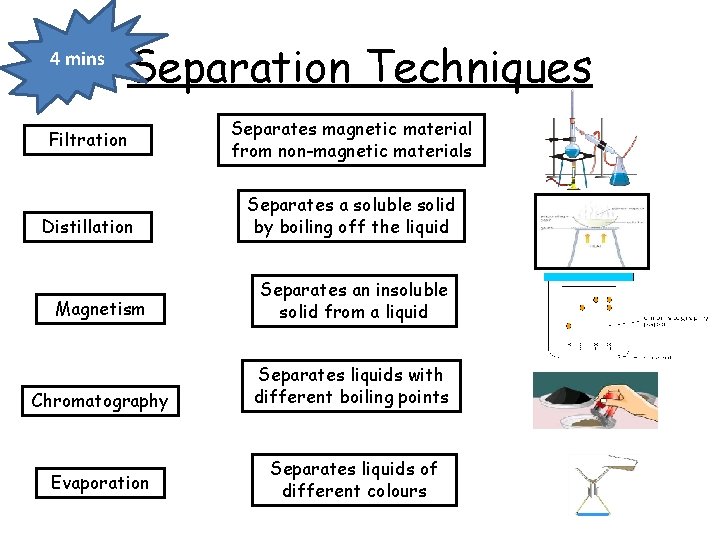
4 mins Separation Techniques Filtration Distillation Separates magnetic material from non-magnetic materials Separates a soluble solid by boiling off the liquid Magnetism Separates an insoluble solid from a liquid Chromatography Separates liquids with different boiling points Evaporation Separates liquids of different colours
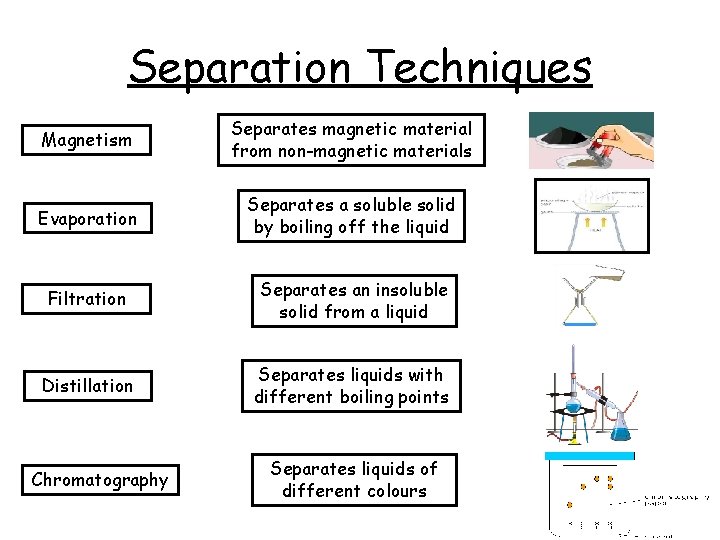
Separation Techniques Magnetism Separates magnetic material from non-magnetic materials Evaporation Separates a soluble solid by boiling off the liquid Filtration Separates an insoluble solid from a liquid Distillation Separates liquids with different boiling points Chromatography Separates liquids of different colours

Particle Models
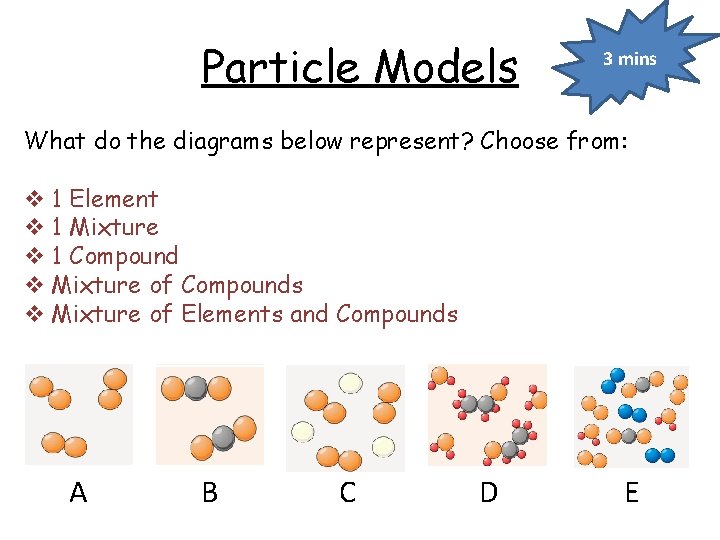
Particle Models 3 mins What do the diagrams below represent? Choose from: v 1 Element v 1 Mixture v 1 Compound v Mixture of Compounds v Mixture of Elements and Compounds A B C D E
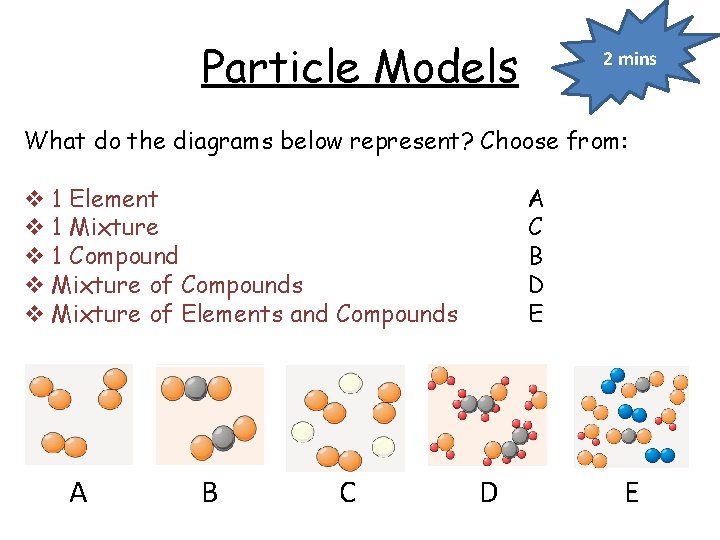
Particle Models 2 mins What do the diagrams below represent? Choose from: v 1 Element v 1 Mixture v 1 Compound v Mixture of Compounds v Mixture of Elements and Compounds A B C A C B D E
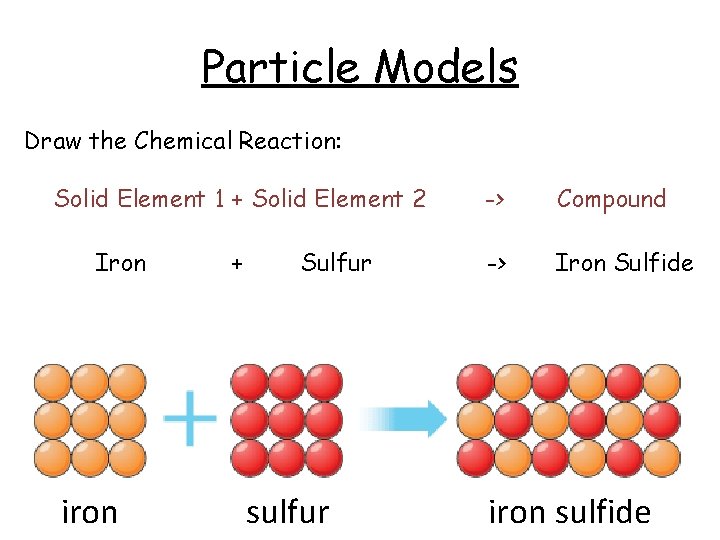
Particle Models Draw the Chemical Reaction: Solid Element 1 + Solid Element 2 Iron iron + Sulfur sulfur -> Compound -> Iron Sulfide iron sulfide
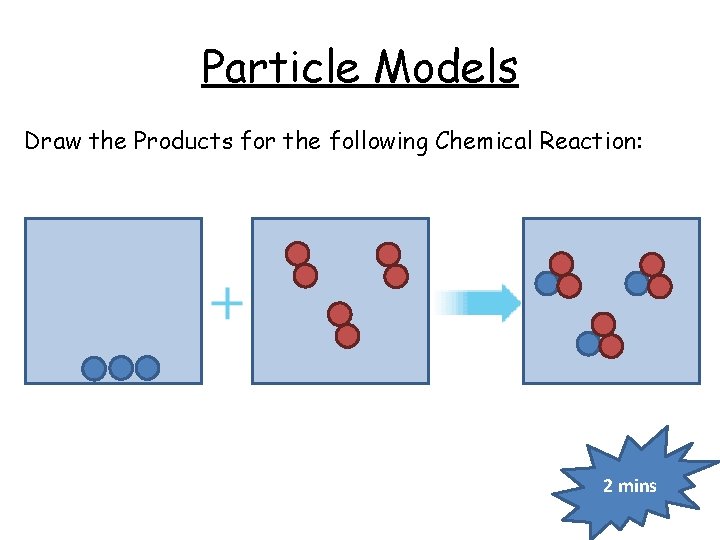
Particle Models Draw the Products for the following Chemical Reaction: 2 mins
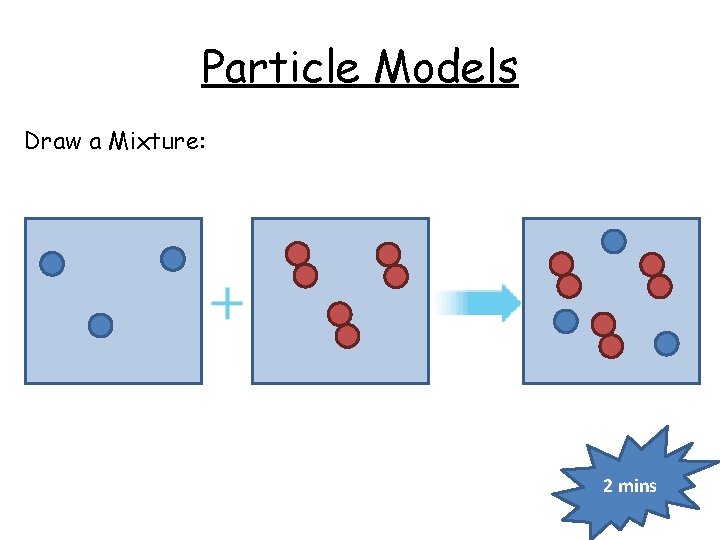
Particle Models Draw a Mixture: 2 mins

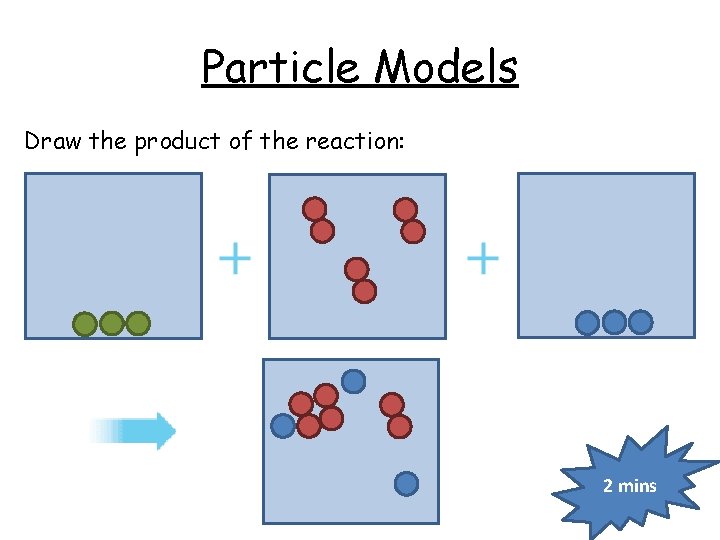
Particle Models Draw the product of the reaction: 2 mins
- Slides: 19