REVISION Lab 6 Solutions Solutions Solution is the

REVISION

Lab ( 6 ) Solutions

Solutions Solution: is the homologous mixture of 2 or more substances in different proportion, substances can be solids, liquids or gases or combination of the three.

percentage of aqueous solutions called Tissue fluid and divided into: 1) Intercellular fluid 2)extracellular fluid -These fluids serves in exchanging substances between cell and its surrounding to insure performing vital biological functions of the cell and to maintain cell shape and volume. -Water is the main component of living cell and protoplasm, and the main solvent on living tissues.

Physical and Chemical Properties of Water 1 - Water has broad range between freezing and boiling degrees. 2 - Has the ability to bind to macromolecules such as proteins, carbohydrates and nucleic acids with hydrogen bonds (H-bond). 3 - Has many physical characteristics such as: surface tension, viscosity, cohesion and adhesion. 4 - Polarity of water helps adhesion to surface of many molecular organic components such as: starch, proteins and moisten them and form colloidal solutions. 5 - Water is an ideal solvent of wide range of ionic substances, also to non-ionic substances. 6 - water is inactive chemically. This property provides ideal medium for biochemical reactions such as photosynthesis.

Types of Aqueous Solutions 1 - True Solution: homologous, does not precipitate with time, and filtrate through filter paper such as aqueous sucrose solution and copper sulphate solution. 2 - Suspension: Heterogeneous. Solute particles diffuse into large particles that can be seen by eye or microscope. They do not filtrate through filter paper, and can be easily separated such as aqueous solution of calcium sulphate.

3 - Emulsion: The mixture of 2 immiscible liquids (such as water and oil). One liquid particles suspend in form of tiny globes in the other liquid, and when left aside they separate into two layers. By adding a emulsifying agent (as Na. OH) it helps stabilize the emulsion. 4 - Colloidal solution: Molecules spread between solvent’s particles, and does not precipitate or float. Also they do not filtrate through membranes (animal membranes or artificial membranes such as cellophane), but they can filtrate though regular filter paper. Colloidal solutions are viscous. Solute is called dispersed phase, and the solvent is called dispersion medium. (surface aggregation- Brownian motion- membrane semipermeability- The ability to absorb water )
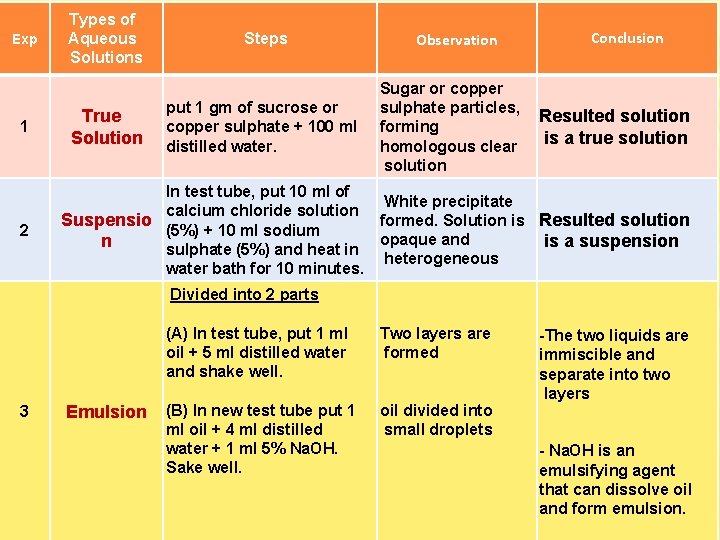
Exp 1 2 Types of Aqueous Solutions True Solution Steps put 1 gm of sucrose or copper sulphate + 100 ml distilled water. Observation Sugar or copper sulphate particles, forming homologous clear solution Conclusion Resulted solution is a true solution In test tube, put 10 ml of White precipitate calcium chloride solution Suspensio formed. Solution is Resulted solution (5%) + 10 ml sodium opaque and n is a suspension sulphate (5%) and heat in heterogeneous water bath for 10 minutes. Divided into 2 parts 3 Emulsion (A) In test tube, put 1 ml oil + 5 ml distilled water and shake well. Two layers are formed (B) In new test tube put 1 ml oil + 4 ml distilled water + 1 ml 5% Na. OH. Sake well. oil divided into small droplets -The two liquids are immiscible and separate into two layers - Na. OH is an emulsifying agent that can dissolve oil and form emulsion.
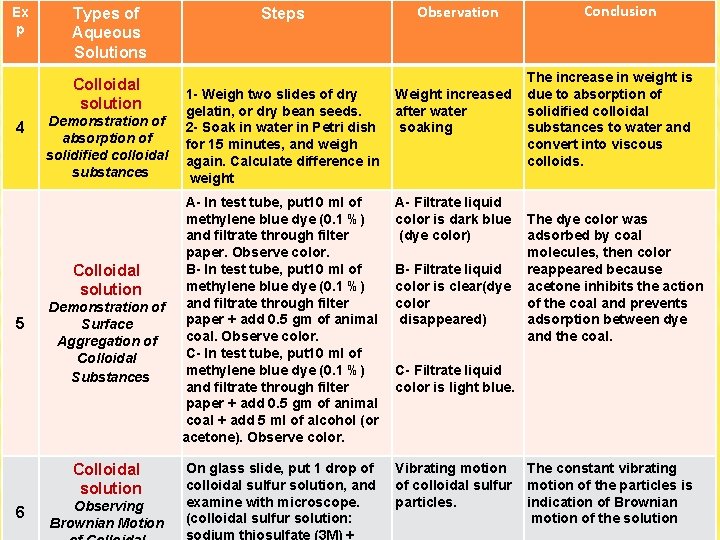
Ex p 4 5 Types of Aqueous Solutions Steps Colloidal solution 1 - Weigh two slides of dry gelatin, or dry bean seeds. 2 - Soak in water in Petri dish for 15 minutes, and weigh again. Calculate difference in weight Demonstration of absorption of solidified colloidal substances Weight increased after water soaking A- In test tube, put 10 ml of methylene blue dye (0. 1 %) and filtrate through filter paper. Observe color. B- In test tube, put 10 ml of Colloidal methylene blue dye (0. 1 %) solution Demonstration of and filtrate through filter paper + add 0. 5 gm of animal Surface coal. Observe color. Aggregation of C- In test tube, put 10 ml of Colloidal methylene blue dye (0. 1 %) Substances and filtrate through filter paper + add 0. 5 gm of animal coal + add 5 ml of alcohol (or acetone). Observe color. A- Filtrate liquid color is dark blue (dye color) On glass slide, put 1 drop of colloidal sulfur solution, and examine with microscope. (colloidal sulfur solution: Vibrating motion of colloidal sulfur particles. Colloidal solution 6 Observation Observing Brownian Motion B- Filtrate liquid color is clear(dye color disappeared) Conclusion The increase in weight is due to absorption of solidified colloidal substances to water and convert into viscous colloids. The dye color was adsorbed by coal molecules, then color reappeared because acetone inhibits the action of the coal and prevents adsorption between dye and the coal. C- Filtrate liquid color is light blue. The constant vibrating motion of the particles is indication of Brownian motion of the solution

Lab ( 9 ) Diffusion, Osmosis and Photosynthesis
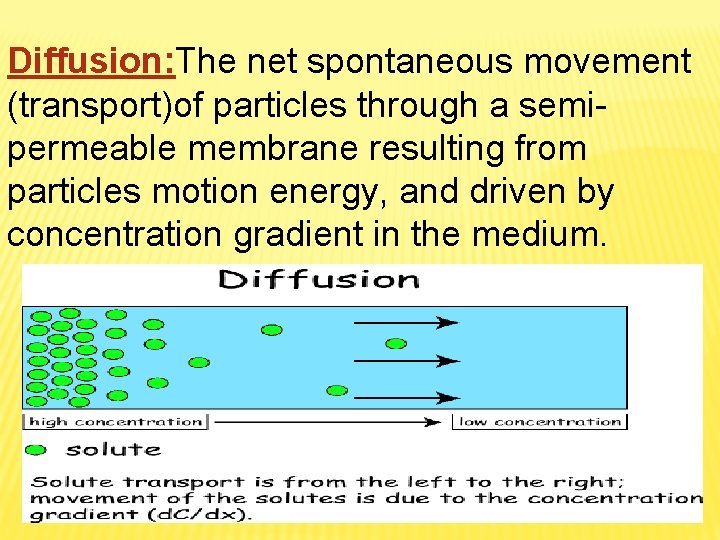
Diffusion: The net spontaneous movement (transport)of particles through a semipermeable membrane resulting from particles motion energy, and driven by concentration gradient in the medium.
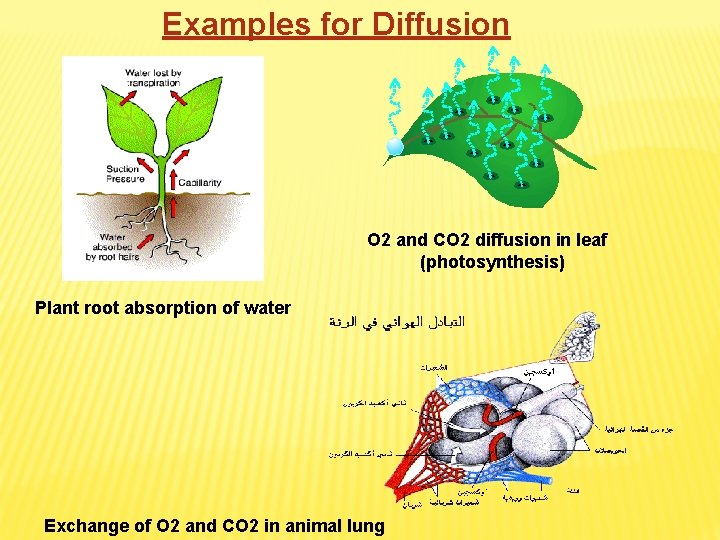
Examples for Diffusion O 2 and CO 2 diffusion in leaf (photosynthesis) Plant root absorption of water Exchange of O 2 and CO 2 in animal lung
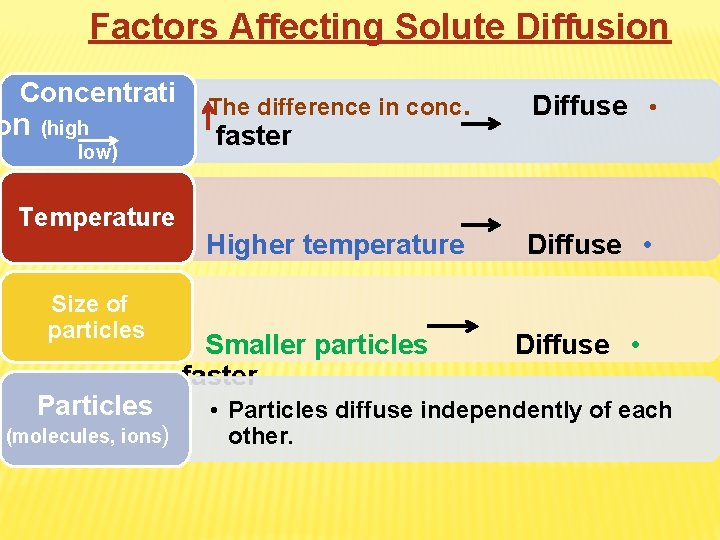
Factors Affecting Solute Diffusion Concentrati on (high low) Temperature Size of particles Particles (molecules, ions) The difference in conc. Diffuse • Higher temperature Diffuse • faster Smaller particles faster Diffuse • • Particles diffuse independently of each other.
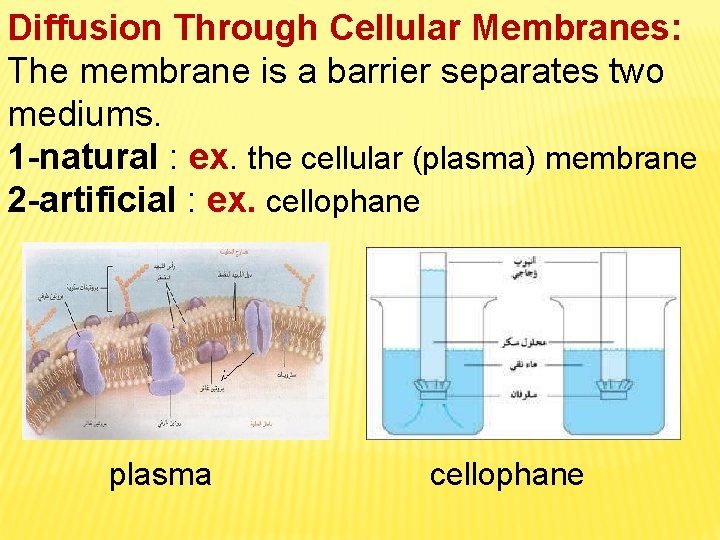
Diffusion Through Cellular Membranes: The membrane is a barrier separates two mediums. 1 -natural : ex. the cellular (plasma) membrane 2 -artificial : ex. cellophane plasma cellophane
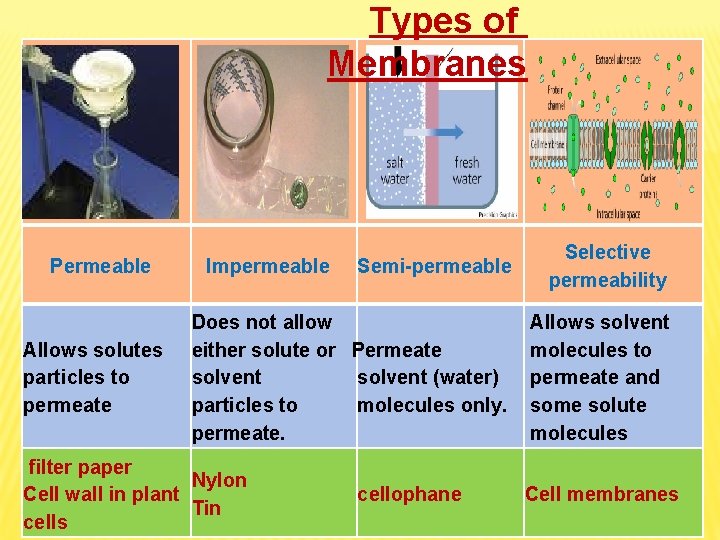
Types of Membranes Permeable Allows solutes particles to permeate Semi-permeable Selective permeability Does not allow either solute or Permeate solvent (water) particles to molecules only. permeate. Allows solvent molecules to permeate and some solute molecules Impermeable filter paper Nylon Cell wall in plant Tin cells cellophane Cell membranes
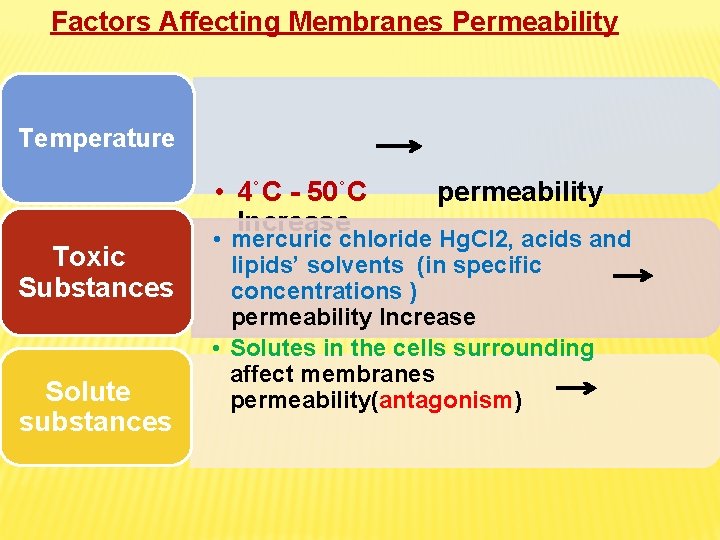
Factors Affecting Membranes Permeability Temperature • 4˚C - 50˚C Increase Toxic Substances Solute substances permeability • mercuric chloride Hg. Cl 2, acids and lipids’ solvents (in specific concentrations ) permeability Increase • Solutes in the cells surrounding affect membranes permeability(antagonism)

Experiments for Diffusions
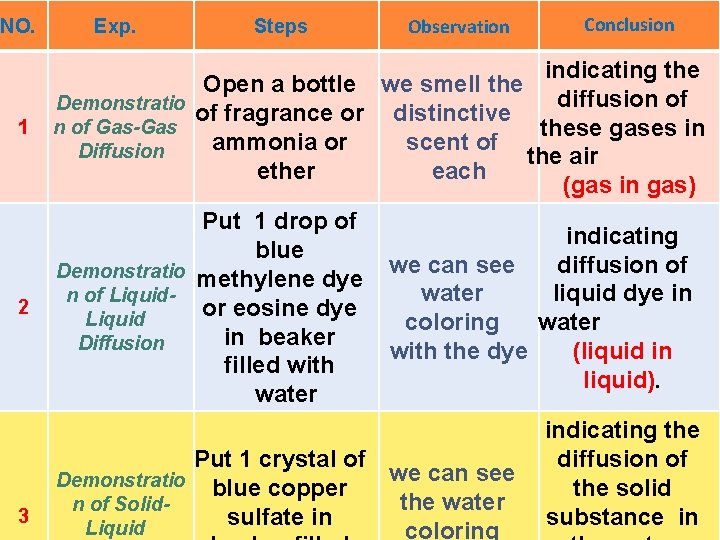
NO. Exp. Steps Observation Conclusion indicating the Open a bottle we smell the diffusion of Demonstratio of fragrance or distinctive 1 n of Gas-Gas these gases in ammonia or scent of Diffusion the air ether each (gas in gas) 2 3 Put 1 drop of blue Demonstratio methylene dye n of Liquidor eosine dye Liquid in beaker Diffusion filled with water indicating we can see diffusion of liquid dye in water coloring water with the dye (liquid in liquid). Put 1 crystal of we can see Demonstratio blue copper the water n of Solidsulfate in Liquid coloring indicating the diffusion of the solid substance in
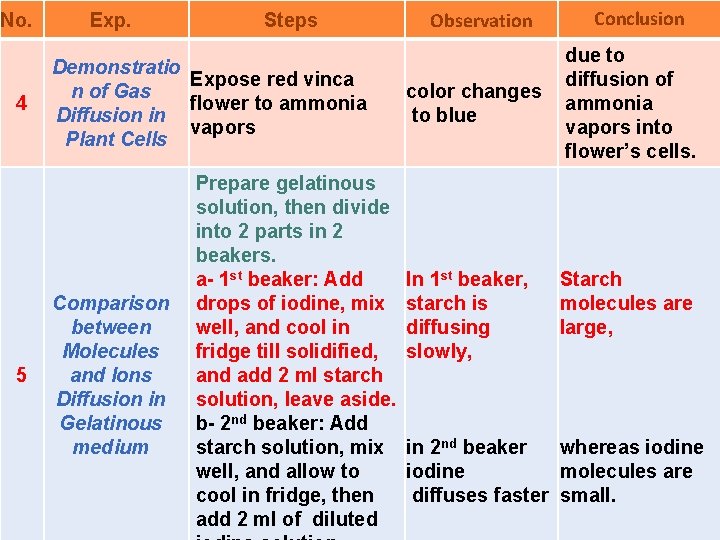
No. 4 5 Exp. Steps Demonstratio Expose red vinca n of Gas flower to ammonia Diffusion in vapors Plant Cells Comparison between Molecules and Ions Diffusion in Gelatinous medium Prepare gelatinous solution, then divide into 2 parts in 2 beakers. a- 1 st beaker: Add drops of iodine, mix well, and cool in fridge till solidified, and add 2 ml starch solution, leave aside. b- 2 nd beaker: Add starch solution, mix well, and allow to cool in fridge, then add 2 ml of diluted Observation color changes to blue In 1 st beaker, starch is diffusing slowly, Conclusion due to diffusion of ammonia vapors into flower’s cells. Starch molecules are large, in 2 nd beaker whereas iodine molecules are diffuses faster small.
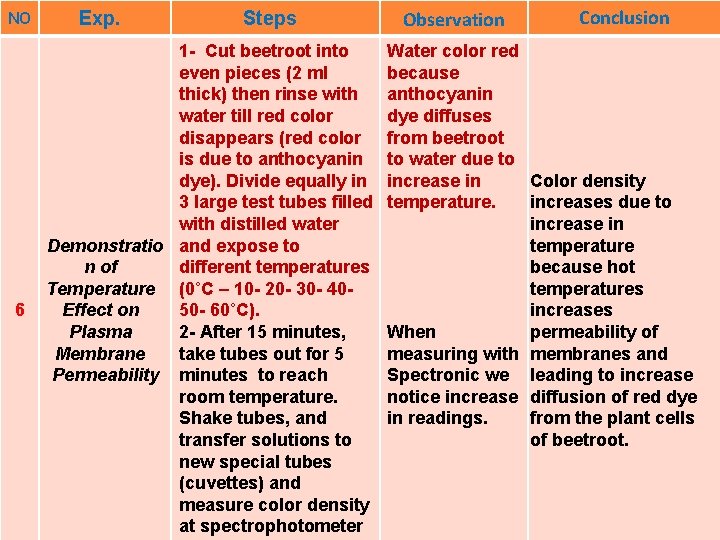
NO 6 Exp. Steps 1 - Cut beetroot into even pieces (2 ml thick) then rinse with water till red color disappears (red color is due to anthocyanin dye). Divide equally in 3 large test tubes filled with distilled water Demonstratio and expose to different temperatures n of Temperature (0˚C – 10 - 20 - 30 - 4050 - 60˚C). Effect on 2 - After 15 minutes, Plasma take tubes out for 5 Membrane Permeability minutes to reach room temperature. Shake tubes, and transfer solutions to new special tubes (cuvettes) and measure color density at spectrophotometer Observation Conclusion Water color red because anthocyanin dye diffuses from beetroot to water due to increase in Color density temperature. increases due to increase in temperature because hot temperatures increases When permeability of measuring with membranes and Spectronic we leading to increase notice increase diffusion of red dye in readings. from the plant cells of beetroot.
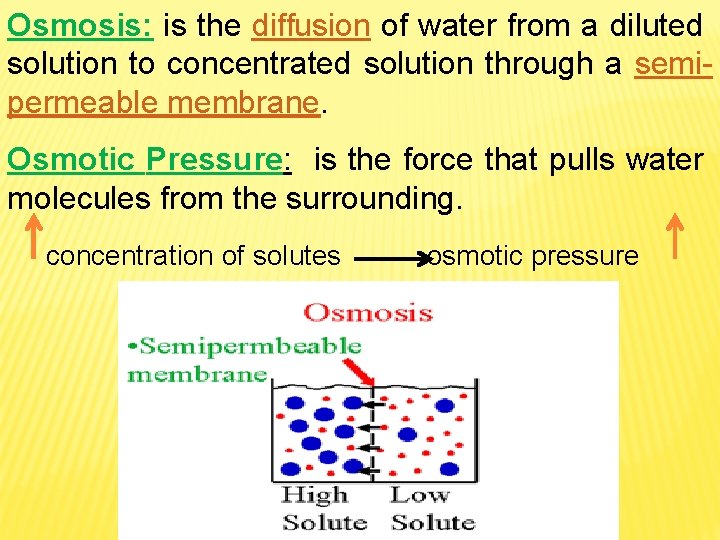
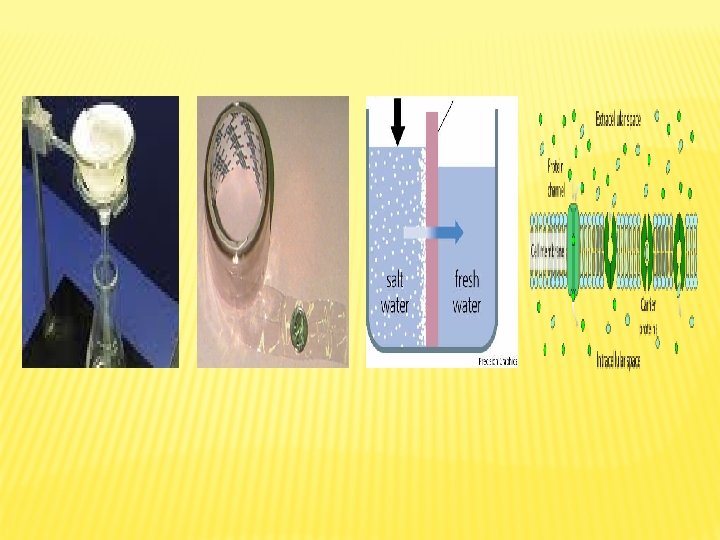
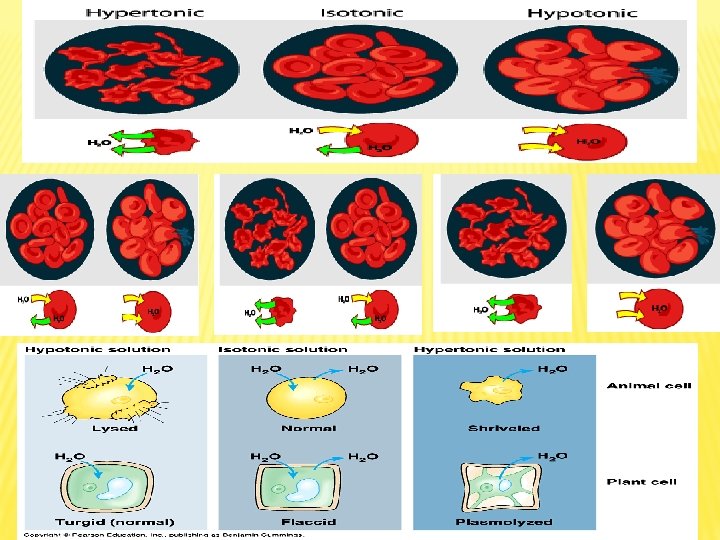
Osmosis: is the diffusion of water from a diluted solution to concentrated solution through a semipermeable membrane. Osmotic Pressure: is the force that pulls water molecules from the surrounding. concentration of solutes osmotic pressure

Types of Solution Concentrations and Osmosis Hypotonic Solution Isotonic solution When living cell is put in distilled water or hypotonic solution, the rate of water entry into cell is higher than water exit, which lead to continuous entry of water inside the cell ( by osmosis) causing cell swelling, and in extreme cases cell explode. When cell is put in isotonic solution, its volume and natural shape do not change because the rate of water entry into the cell equals the rate of exit. Isotonic solution concentration equals the intracellular concentration. Hypertonic solution When cell is put in saline or hypertonic solution (high concentration solution), the rate of water exit from the cell is higher than the rate of entry (by osmosis), this leads to cell shrinking. This process is known
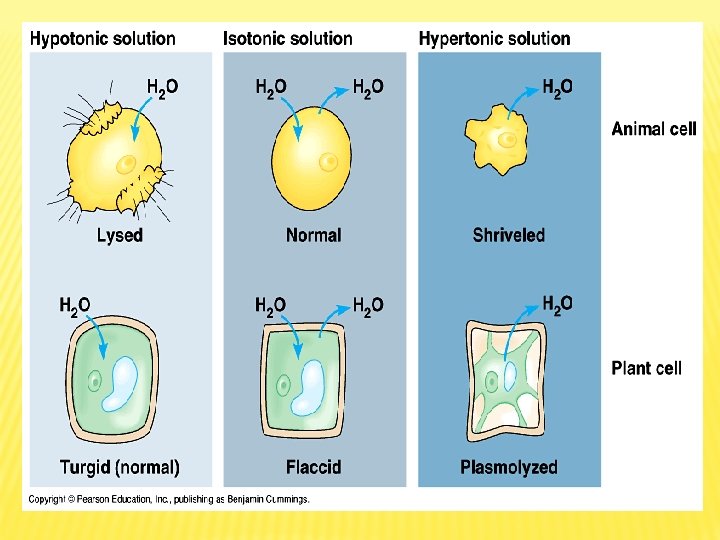
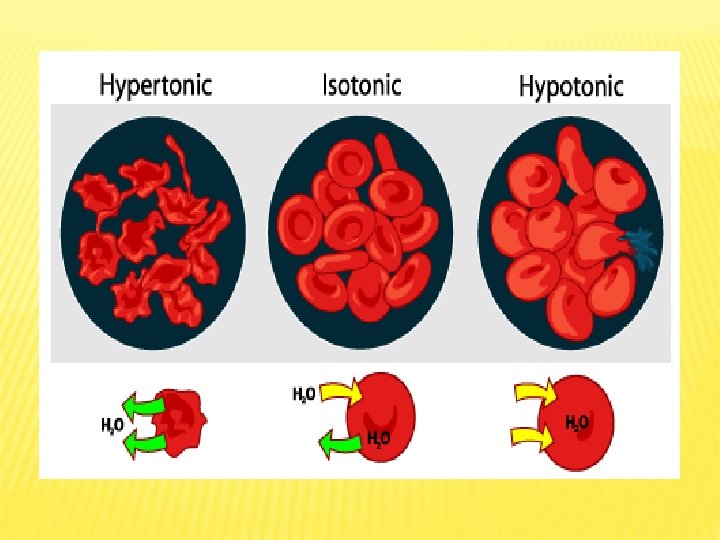

Experiments on Osmosis
N O 1 2 Exp. Steps Cut tips of freshly grown castor plant leaves horizontally (vertically) and Demonstratio identify cortex and n of Osmosis derma. Then immerse by Tropism some of them in distilled water, and some in 10% saline solution for 10 minutes Demonstratio n of decrease weight Due to Plasmolysis, and Increase weight Due to Swelling( by Osmosis) Cut a potato into small pieces and weigh. Divide into 2 parts. -Put one part in saline solution (10%) for 15 minutes, dry and weigh again. -Put the other part in distilled water for 15 minutes, then dry and weigh again. Observation Conclusion Leaf tips in distilled water swollen and bend (tropism) with cortex to the outer side and derma in the inside. Tips in saline solution bend with cortex inside and derma to the outside. Distilled water is a hypotonic solution because its concentration is less than intracellular solution, and water move to the inside of the cell. Whereas the saline solution is a hypertonic solution and water moves from the inside of the cell to outside. -Potato part that was put in saline solution decrease in weight, --whereas the part put in distilled water increase weight. -Distilled water is a hypotonic solution because its concentration is less than potato cells, so water moves into the cell and increase its weight. -Whereas saline solution is a hypertonic solution, its concentration is higher than that of the cell, so water exit from the cell to
No Exp. Steps Observation Conclusion Cleanse a finger with alcohol swab. With a sterile lancet, make a puncture on a fingertip. Apply 3 drop of blood on 3 clean glass slides and make smear: 3 1 st slide is a Demonstratio “control”, examine n of Effect of RBCs under Extracellular microscope. Solution on Red Blood Cells (RBCs) 2 nd slide: mix blood with drops of distilled water, examine under microscope. 3 rd slide: mix blood with drops of 5% In control, normal RBCs shape and size because they are in normal isotonic RBCs that are mixed with distilled water swollen (increase volum), RBCs in Na. Cl solution shrunk (decrease volume). the rate of water entry into the cell equals the rate of exit. the rate of water entry into cell is higher than water exit, the rate of water exit from the cell is higher than

Photosynthesis: Green plants and some protozoa (cyanobacteria and algae) can build their own nutrients via photosynthesis, They absorb light energy and coverts it into chemical energy, producing organic compounds. Carbon dioxide + water + sunlight = sugar + oxygen

Experiments on Photosynthesis
N O 1 Exp. Steps Demonstrat ion of Photosynth esis by Detecting Starch Formation in Green Leaves Pick fresh green plants on afternoon, boil to kill germs and put in 70% alcohol to extract pigments till leaves become white and transfer to cold water (to regain lost water) and add a drop of diluted iodine Observation Conclusion Blue color appear on leaves The blue color is indication of starch formation distributed in the leaf
Assignment (9): Fill in the table with your results: 1) Diffusion Experiment: Experiment Gas-gas diffusion Liquid-liquid diffusion Solid-liquid diffusion Gas diffusion in plant cells Comarison of molecules and ions diffusion in gelatinous medium Observation Conclusion


- Put ( T ) for true statements and ( F ) for false statements:

-Fill in the blanks with the correct word to complete the following phrases

- From the data given, how to prepare the following issues. SHOW ME YOUR CALCULATIONS AND PLEASE DO NOT FORGET UNITS!!).
3 -Compare : Types of definition Example
- fill the spaces with the required: NO 1 2 The experiment Steps Observation Conclusion
- Do the experiment then fill the table. NO. The experiment Observation Conclusion


- BONUS:

- Slides: 43