REVISION INTERATOMIC BONDS LEWIS STRUCTURES of covalent molecules

REVISION INTERATOMIC BONDS

LEWIS STRUCTURES of covalent molecules
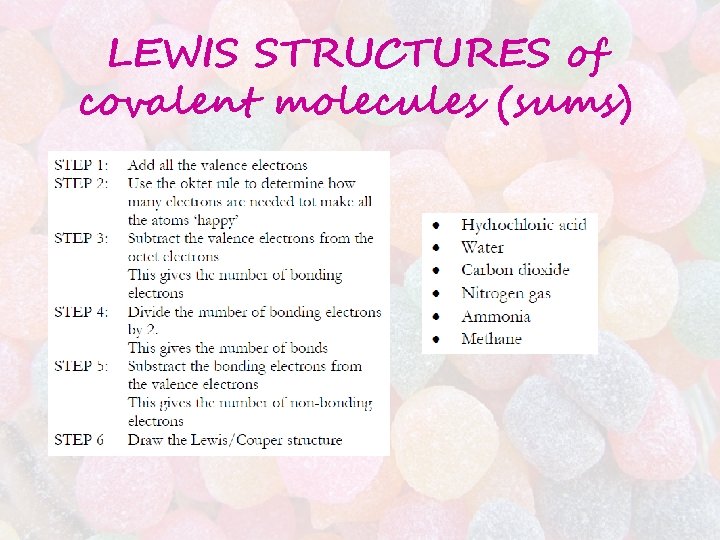
LEWIS STRUCTURES of covalent molecules (sums)
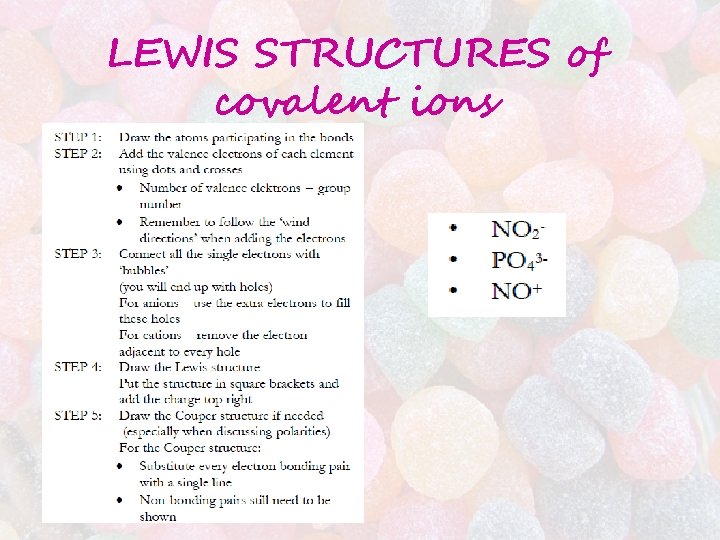
LEWIS STRUCTURES of covalent ions

LEWIS STRUCTURES of DATIVE covalent molecules

LEWIS STRUCTURES with RESONANCE STRUCTURES
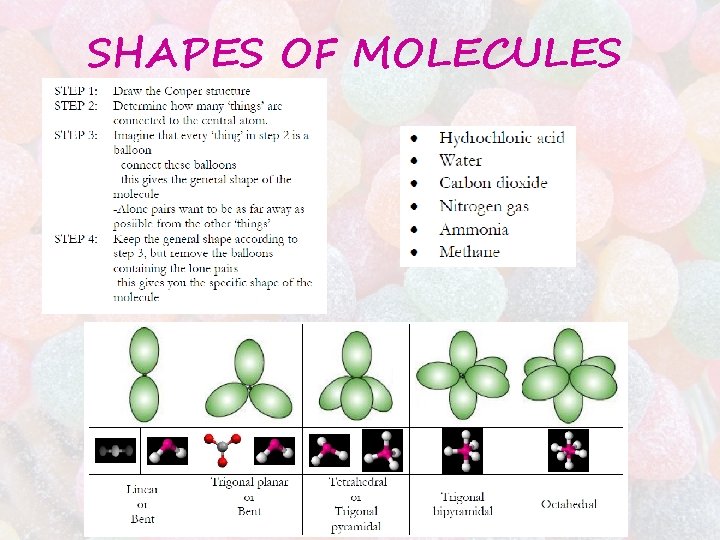
SHAPES OF MOLECULES
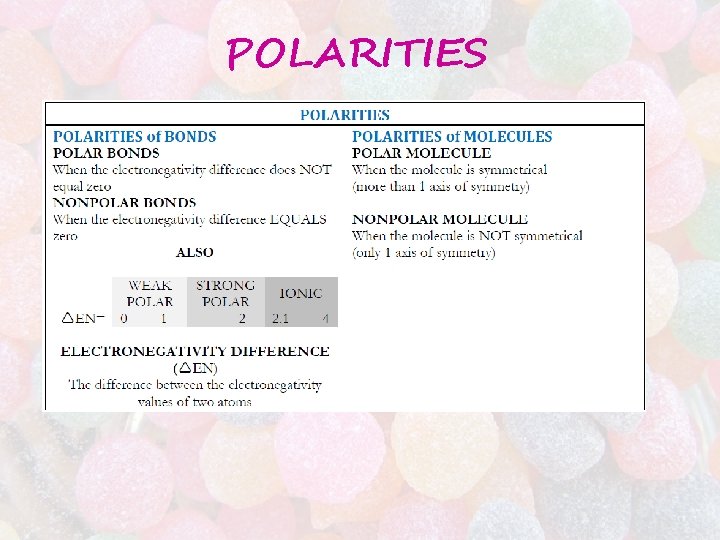
POLARITIES

REVISION INTERMOLECULAR FORCES

VAN DER WAALS FORCES

BOILING POINTS Stronger forces = highest boiling points, because more energy is needed to break the bonds Increases with size Because van der Waals forces increases with size, therefore longer chains have stronger forces and need more energy to break the bonds Decreases with brancing, because branched chains cannot fit tightly together, therefore they are further apart and the van dier Waals forces weaker. Less energy is neede tot break the bonds VAPOUR PRESSURE (how easily it evapourates) Decrease with size, because van der Waals force increase with size Long chaings have stonger forces, therefore more energy is needed to break the bonds and so longer chains take longer to evapourate
VISCOSITY Resistance to flow (how sticky it is) Increases with size, because van der Waals forces increase with size Long chains have stronger forces and will therefore have a high viscosity Die teenstand teen vloei ( of hoe taai dit is)
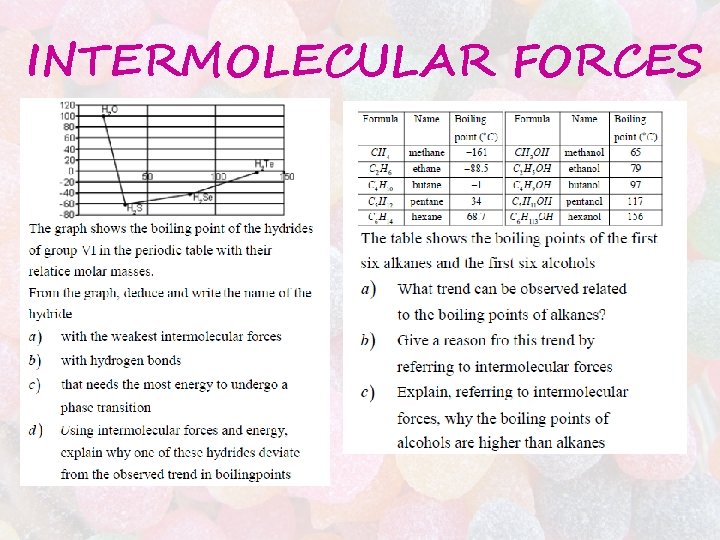
INTERMOLECULAR FORCES
- Slides: 13