Review of Science 10 Dissociation and Word Equations









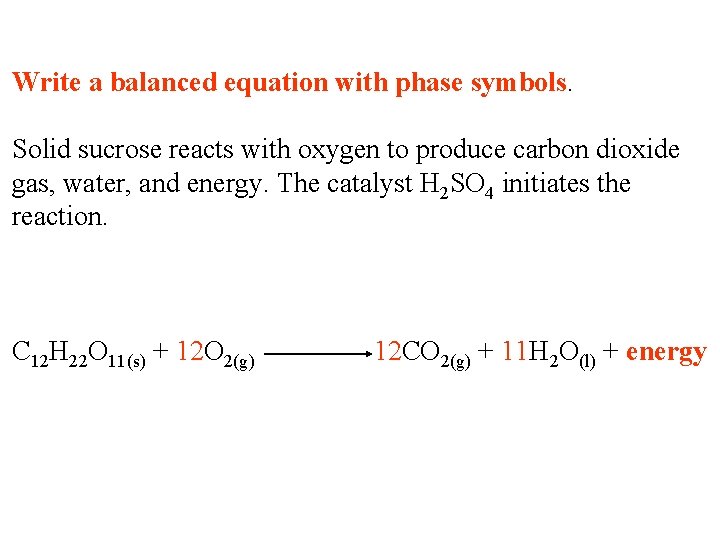
- Slides: 42

Review of Science 10 Dissociation and Word Equations

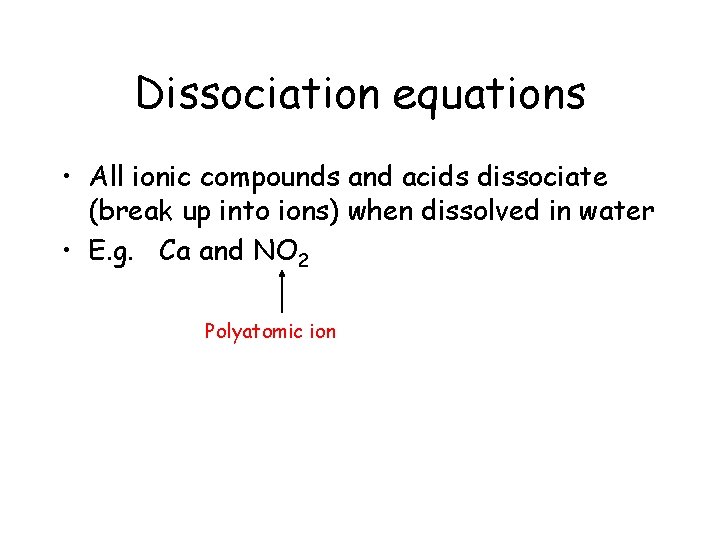
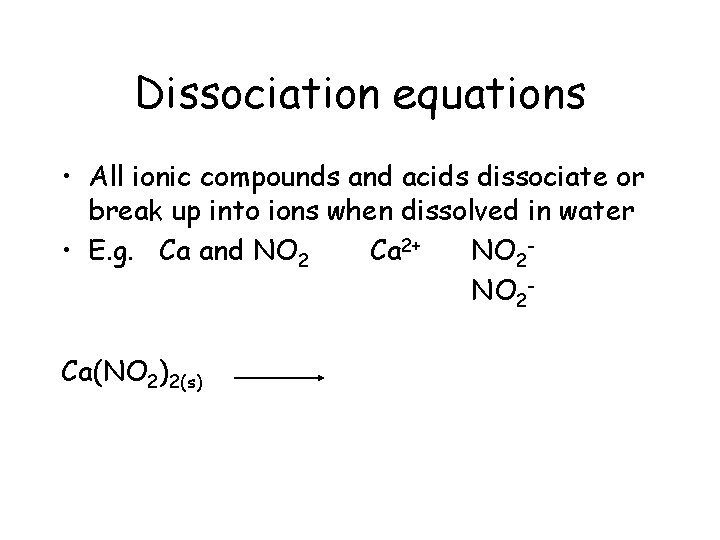
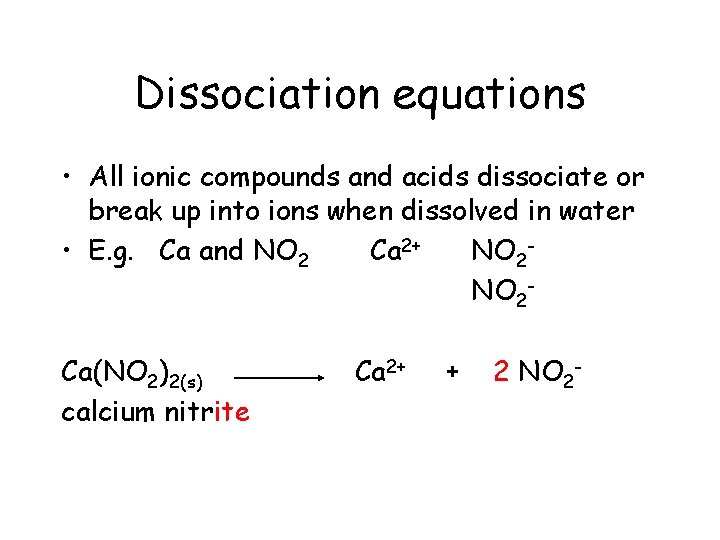
Dissociation equations • All ionic compounds and acids dissociate (break up into ions) when dissolved in water • E. g. Ca and NO 2 Polyatomic ion

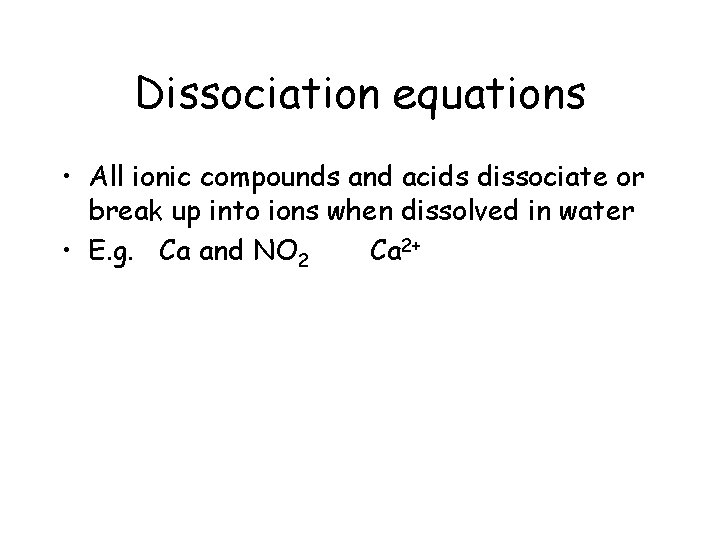
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+

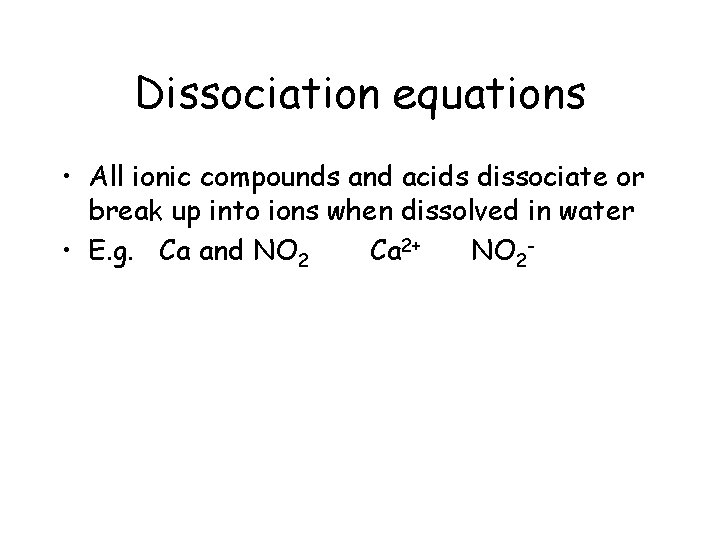
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 -

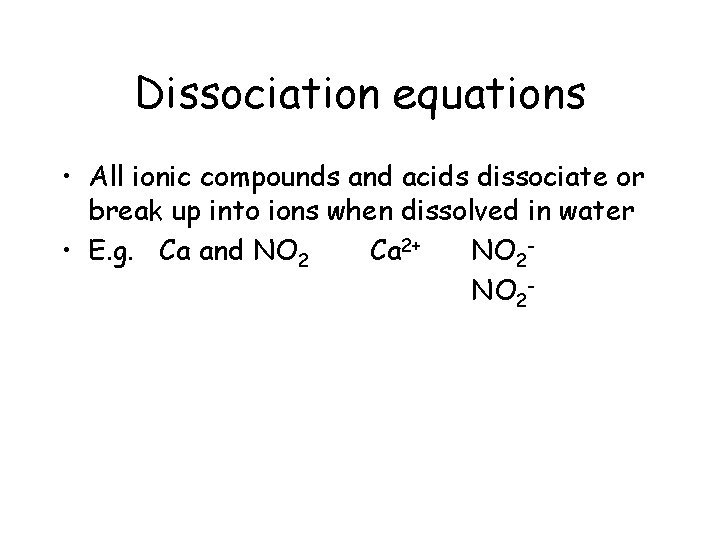
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 -

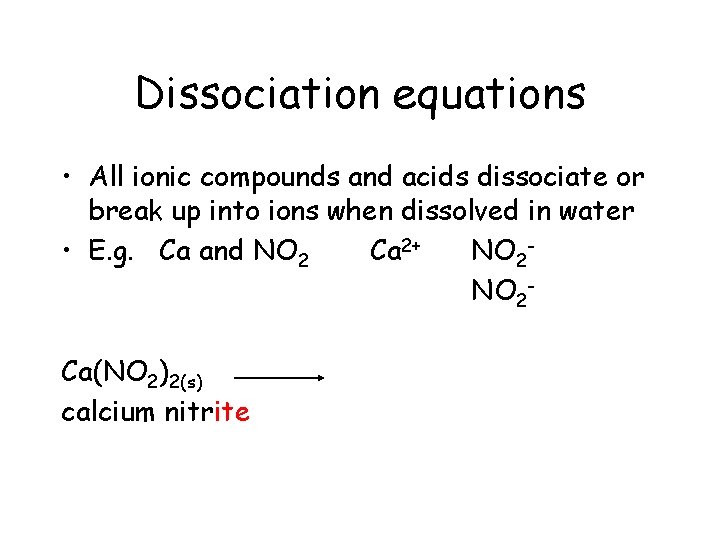
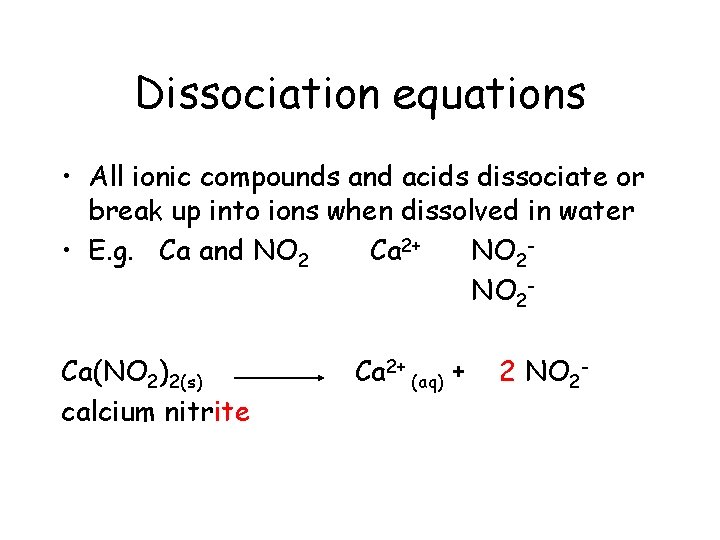
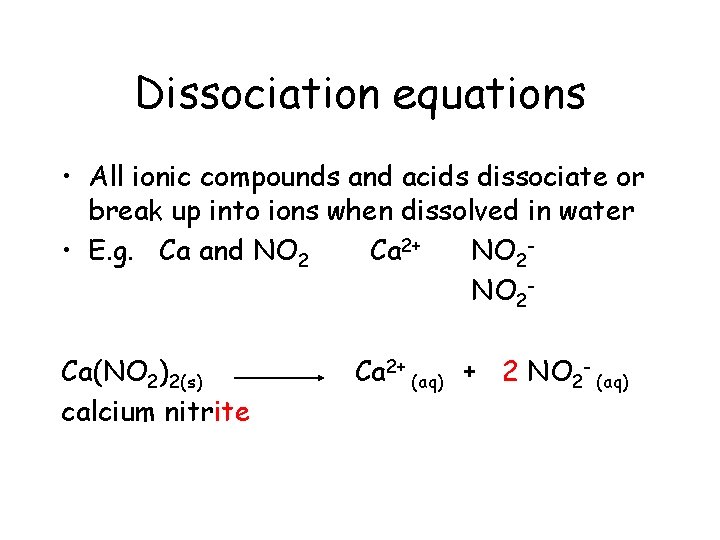
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s)

Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite

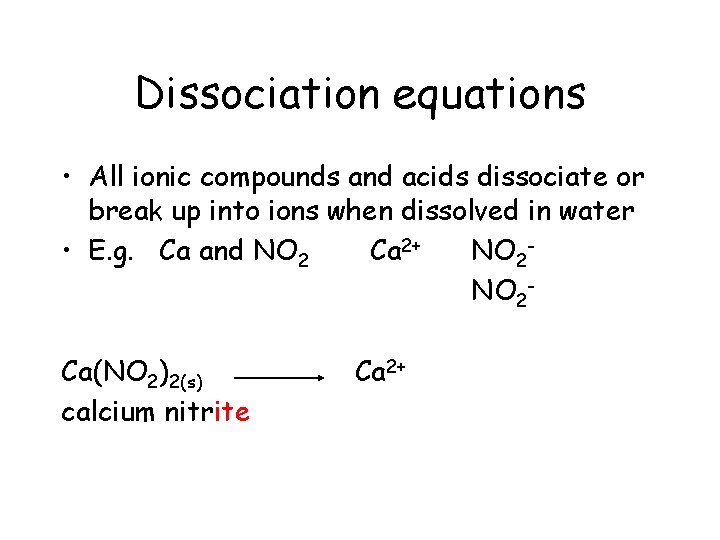
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+

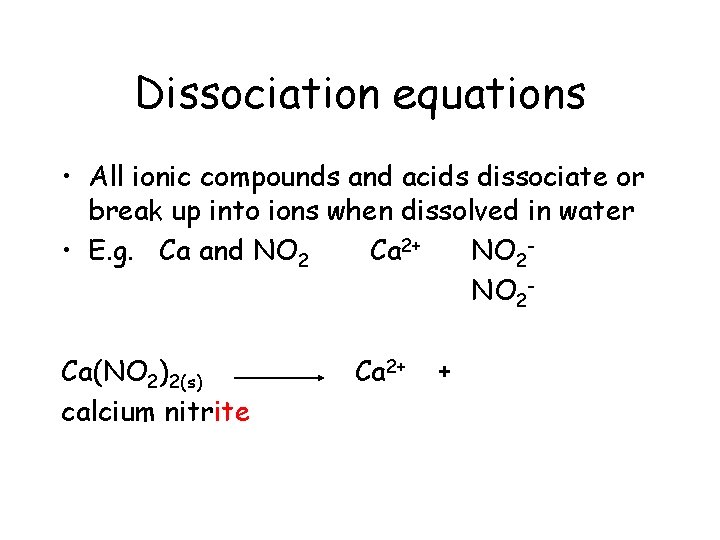
Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+ +

Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+ + 2 NO 2 -

Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+ (aq) + 2 NO 2 -

Dissociation equations • All ionic compounds and acids dissociate or break up into ions when dissolved in water • E. g. Ca and NO 2 Ca 2+ NO 2 Ca(NO 2)2(s) calcium nitrite Ca 2+ (aq) + 2 NO 2 - (aq)

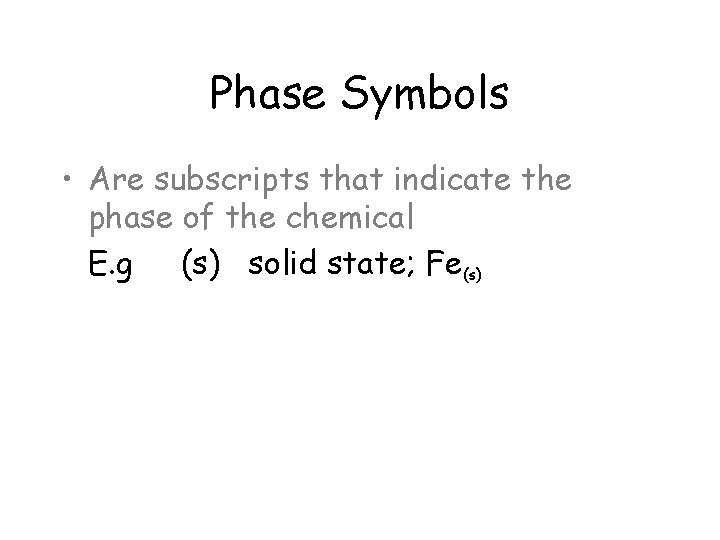
Phase Symbols • Are subscripts that indicate the phase of the chemical

Phase Symbols • Are subscripts that indicate the phase of the chemical E. g (s) solid state; Fe(s)

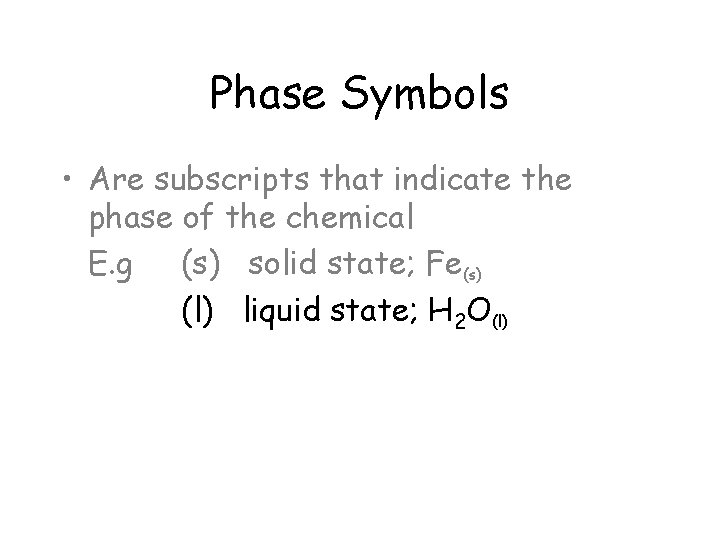
Phase Symbols • Are subscripts that indicate the phase of the chemical E. g (s) solid state; Fe(s) (l) liquid state; H 2 O(l)

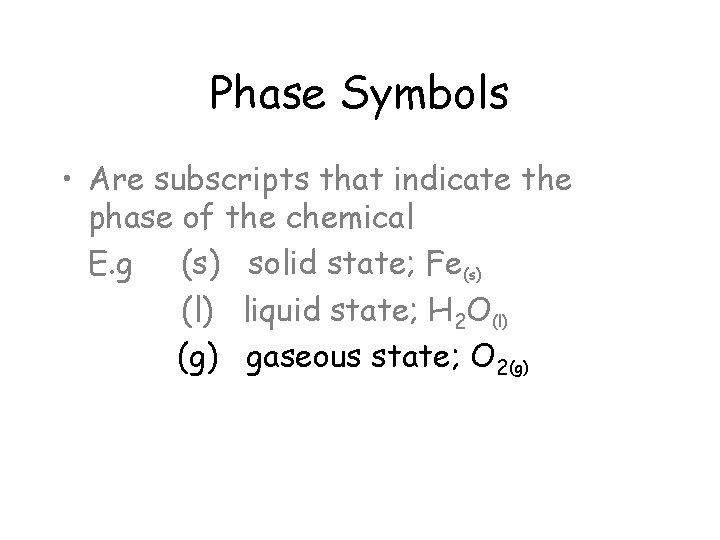
Phase Symbols • Are subscripts that indicate the phase of the chemical E. g (s) solid state; Fe(s) (l) liquid state; H 2 O(l) (g) gaseous state; O 2(g)

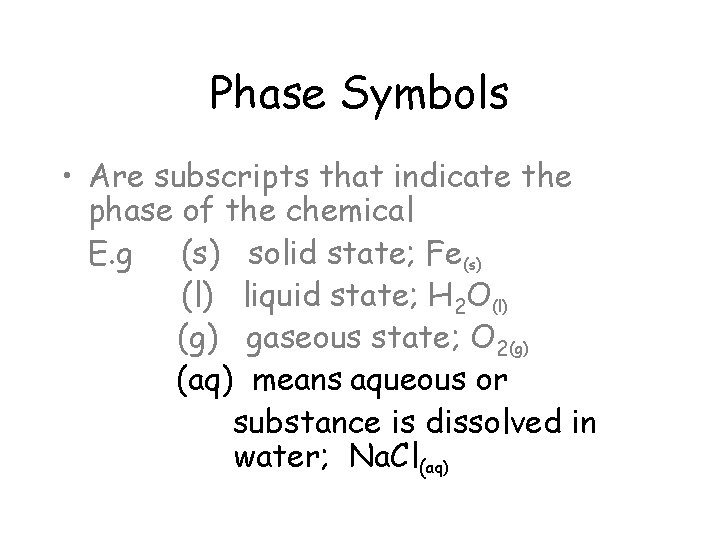
Phase Symbols • Are subscripts that indicate the phase of the chemical E. g (s) solid state; Fe(s) (l) liquid state; H 2 O(l) (g) gaseous state; O 2(g) (aq) means aqueous or substance is dissolved in water; Na. Cl(aq)

Chemical equations from Word Equations

Chemical equations from Word Equations • In Chemistry 11, a solution means something is dissolved in water. Therefore, the phase is aqueous.

Chemical equations from Word Equations • In Chemistry 11, a solution means something is dissolved in water. Therefore, the phase is aqueous. • Must include phase symbols and balance the equation

Chemical equations from Word Equations • In Chemistry 11, a solution means something is dissolved in water. Therefore the phase is aqueous. • Must include phase symbols and balance the equation • Diatomic molecules: H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2


Chemical equations from Word Equations

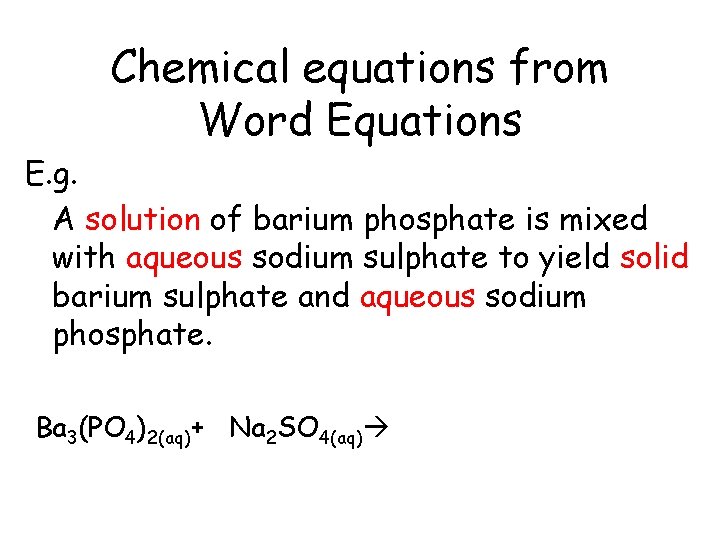
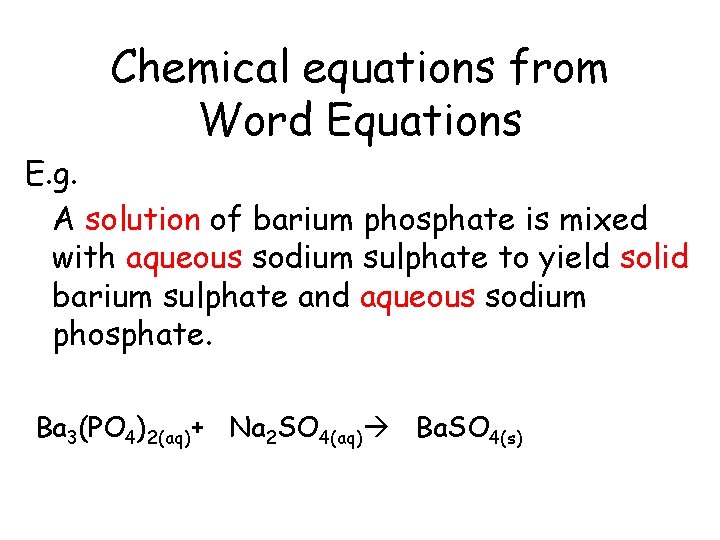
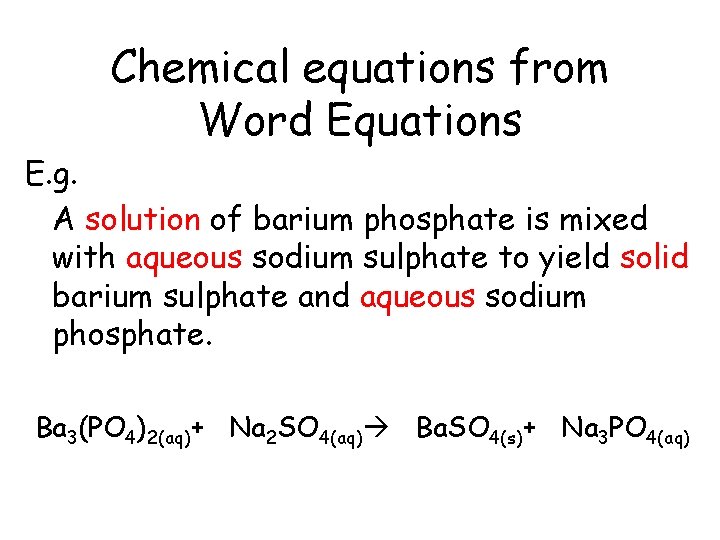
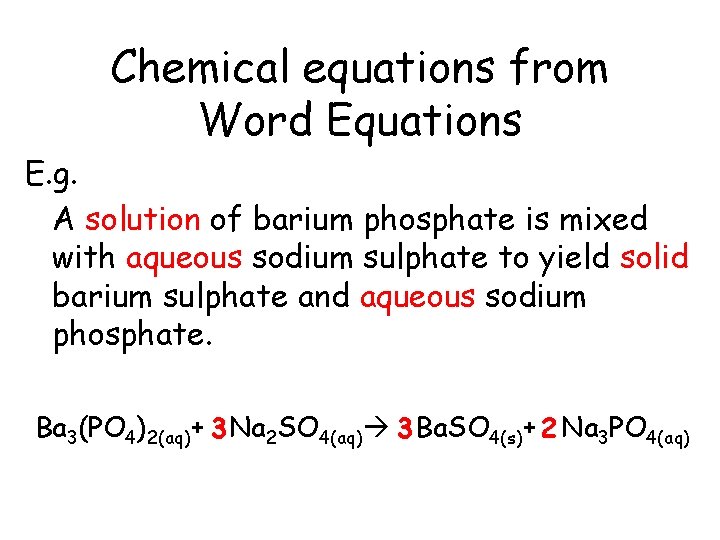
Chemical equations from Word Equations E. g. A solution of barium phosphate is mixed with aqueous sodium sulphate to yield solid barium sulphate and aqueous sodium phosphate.

Chemical equations from Word Equations E. g. A solution of barium phosphate is mixed with aqueous sodium sulphate to yield solid barium sulphate and aqueous sodium phosphate.

Chemical equations from Word Equations E. g. A solution of barium phosphate is mixed with aqueous sodium sulphate to yield solid barium sulphate and aqueous sodium phosphate. Ba 3(PO 4)2(aq)

Chemical equations from Word Equations E. g. A solution of barium phosphate is mixed with aqueous sodium sulphate to yield solid barium sulphate and aqueous sodium phosphate. Ba 3(PO 4)2(aq)+ Na 2 SO 4(aq)

Chemical equations from Word Equations E. g. A solution of barium phosphate is mixed with aqueous sodium sulphate to yield solid barium sulphate and aqueous sodium phosphate. Ba 3(PO 4)2(aq)+ Na 2 SO 4(aq) Ba. SO 4(s)

Chemical equations from Word Equations E. g. A solution of barium phosphate is mixed with aqueous sodium sulphate to yield solid barium sulphate and aqueous sodium phosphate. Ba 3(PO 4)2(aq)+ Na 2 SO 4(aq) Ba. SO 4(s)+ Na 3 PO 4(aq)

Chemical equations from Word Equations E. g. A solution of barium phosphate is mixed with aqueous sodium sulphate to yield solid barium sulphate and aqueous sodium phosphate. Ba 3(PO 4)2(aq)+ 3 Na 2 SO 4(aq) 3 Ba. SO 4(s)+ 2 Na 3 PO 4(aq)

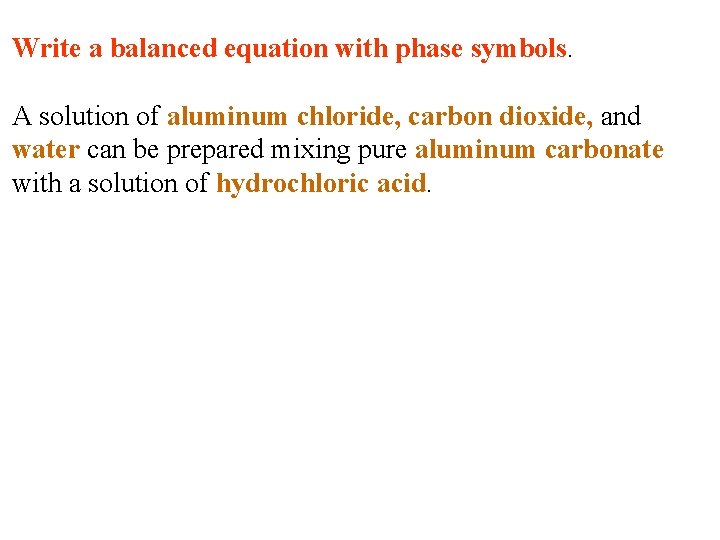
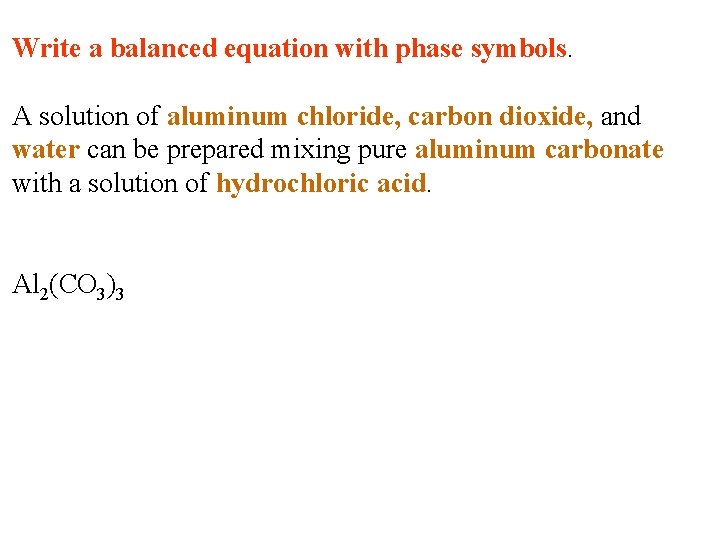
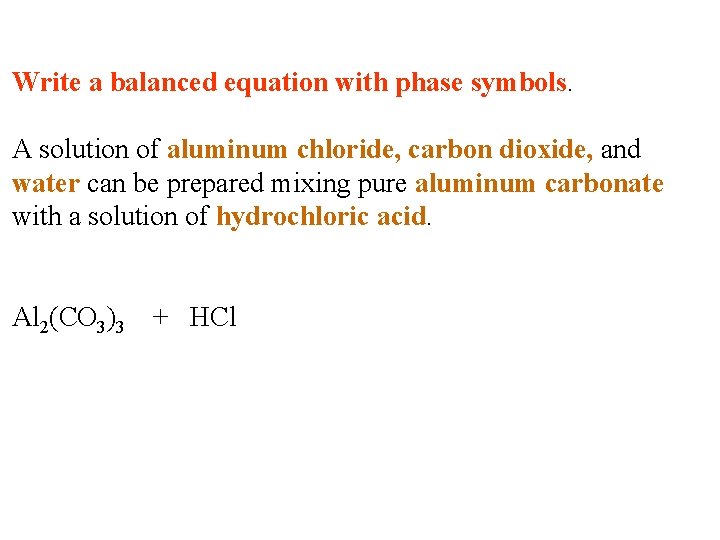
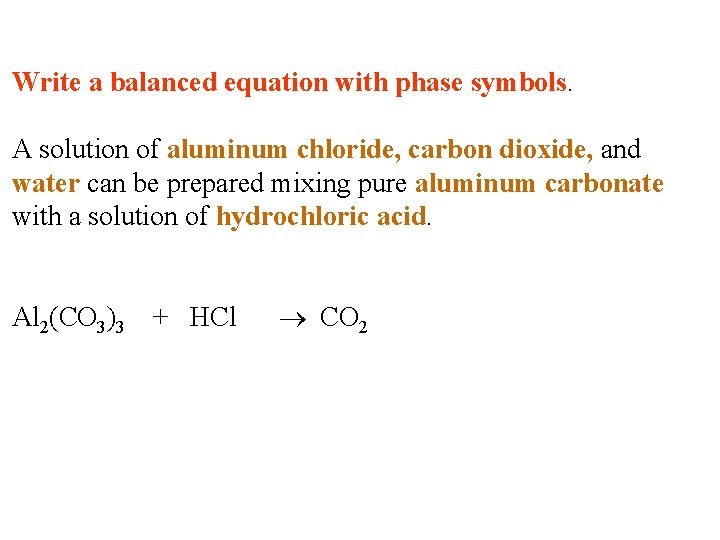
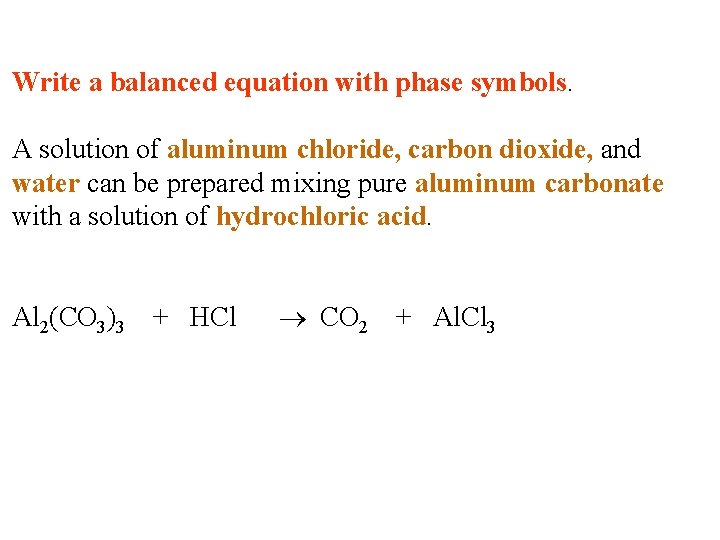
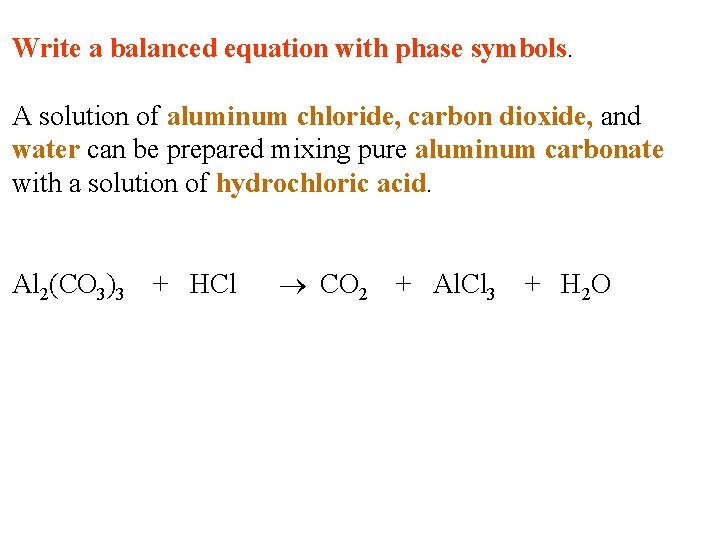
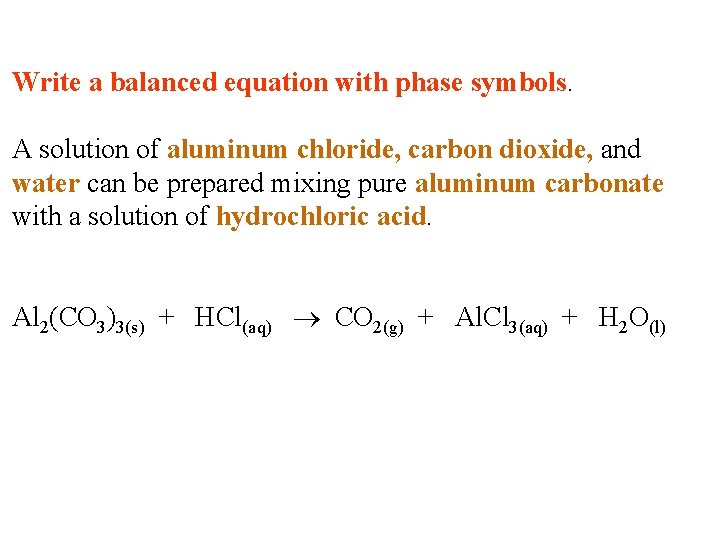
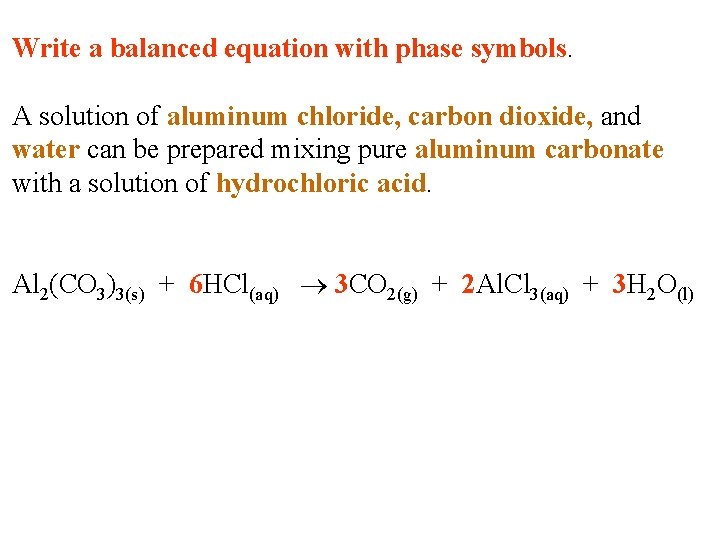
Write a balanced equation with phase symbols. A solution of aluminum chloride, carbon dioxide, and water can be prepared mixing pure aluminum carbonate with a solution of hydrochloric acid.

Write a balanced equation with phase symbols. A solution of aluminum chloride, carbon dioxide, and water can be prepared mixing pure aluminum carbonate with a solution of hydrochloric acid. Al 2(CO 3)3

Write a balanced equation with phase symbols. A solution of aluminum chloride, carbon dioxide, and water can be prepared mixing pure aluminum carbonate with a solution of hydrochloric acid. Al 2(CO 3)3 + HCl

Write a balanced equation with phase symbols. A solution of aluminum chloride, carbon dioxide, and water can be prepared mixing pure aluminum carbonate with a solution of hydrochloric acid. Al 2(CO 3)3 + HCl CO 2

Write a balanced equation with phase symbols. A solution of aluminum chloride, carbon dioxide, and water can be prepared mixing pure aluminum carbonate with a solution of hydrochloric acid. Al 2(CO 3)3 + HCl CO 2 + Al. Cl 3

Write a balanced equation with phase symbols. A solution of aluminum chloride, carbon dioxide, and water can be prepared mixing pure aluminum carbonate with a solution of hydrochloric acid. Al 2(CO 3)3 + HCl CO 2 + Al. Cl 3 + H 2 O

Write a balanced equation with phase symbols. A solution of aluminum chloride, carbon dioxide, and water can be prepared mixing pure aluminum carbonate with a solution of hydrochloric acid. Al 2(CO 3)3(s) + HCl(aq) CO 2(g) + Al. Cl 3(aq) + H 2 O(l)

Write a balanced equation with phase symbols. A solution of aluminum chloride, carbon dioxide, and water can be prepared mixing pure aluminum carbonate with a solution of hydrochloric acid. Al 2(CO 3)3(s) + 6 HCl(aq) 3 CO 2(g) + 2 Al. Cl 3(aq) + 3 H 2 O(l)

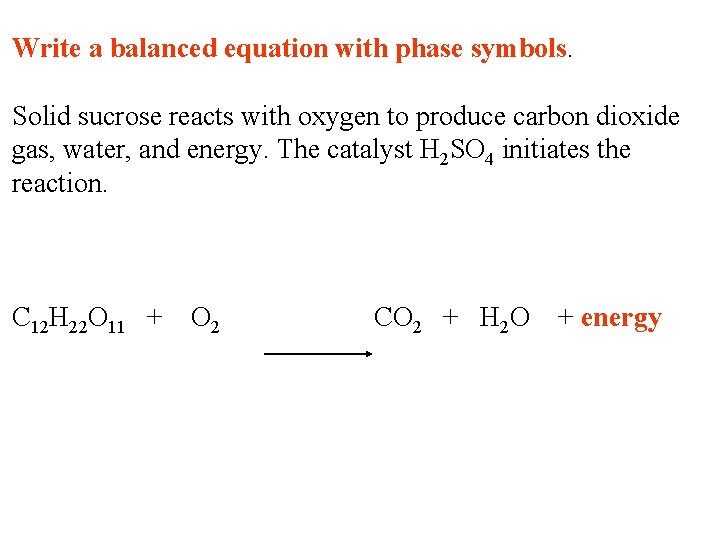
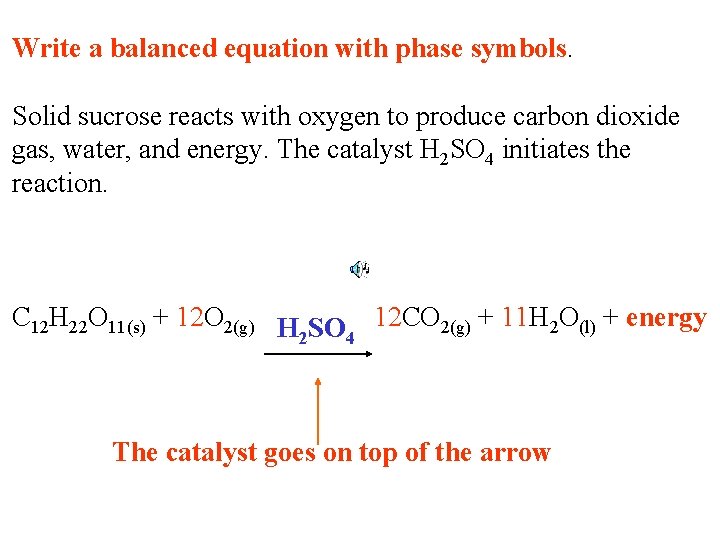
Write a balanced equation with phase symbols. Solid sucrose reacts with oxygen to produce carbon dioxide gas, water, and energy. The catalyst H 2 SO 4 initiates the reaction. C 12 H 22 O 11 + O 2 CO 2 + H 2 O + energy

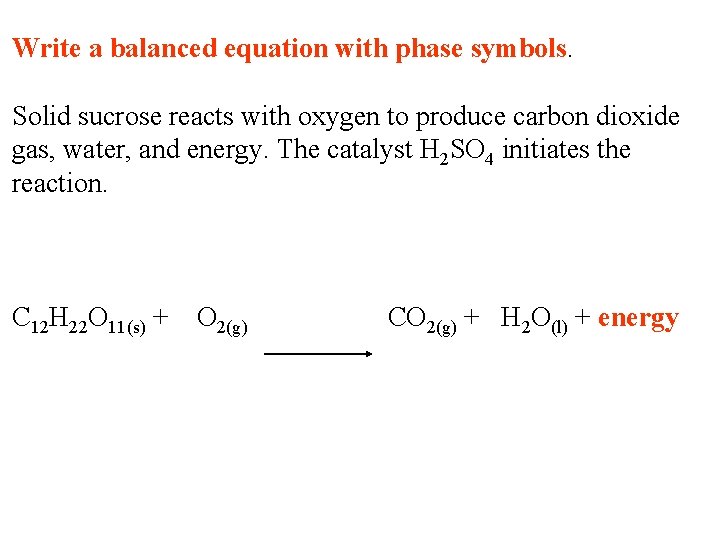
Write a balanced equation with phase symbols. Solid sucrose reacts with oxygen to produce carbon dioxide gas, water, and energy. The catalyst H 2 SO 4 initiates the reaction. C 12 H 22 O 11(s) + O 2(g) CO 2(g) + H 2 O(l) + energy
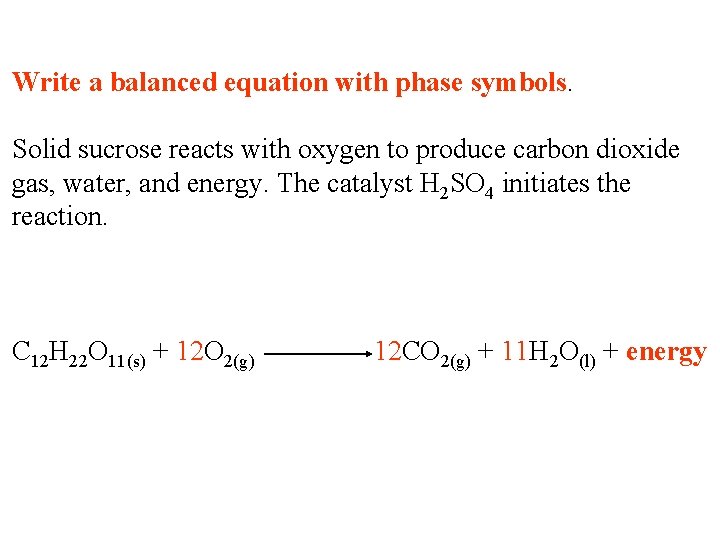
Write a balanced equation with phase symbols. Solid sucrose reacts with oxygen to produce carbon dioxide gas, water, and energy. The catalyst H 2 SO 4 initiates the reaction. C 12 H 22 O 11(s) + 12 O 2(g) 12 CO 2(g) + 11 H 2 O(l) + energy

Write a balanced equation with phase symbols. Solid sucrose reacts with oxygen to produce carbon dioxide gas, water, and energy. The catalyst H 2 SO 4 initiates the reaction. C 12 H 22 O 11(s) + 12 O 2(g) H SO 12 CO 2(g) + 11 H 2 O(l) + energy 2 4 The catalyst goes on top of the arrow