Review of Organic Functional Groups Functional Group Analysis
- Slides: 9

Review of Organic Functional Groups (Functional Group Analysis) Chapter 11 Carboxylic Acids Copyright © 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins

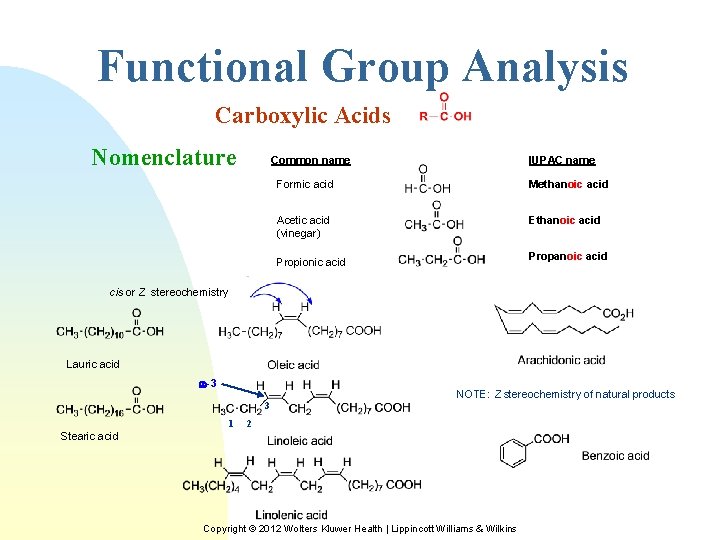
Functional Group Analysis Carboxylic Acids Nomenclature Common name IUPAC name Formic acid Methanoic acid Acetic acid (vinegar) Ethanoic acid Propionic acid cis or Z stereochemistry Lauric acid w-3 3 1 NOTE: Z stereochemistry of natural products 2 Stearic acid Copyright © 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins

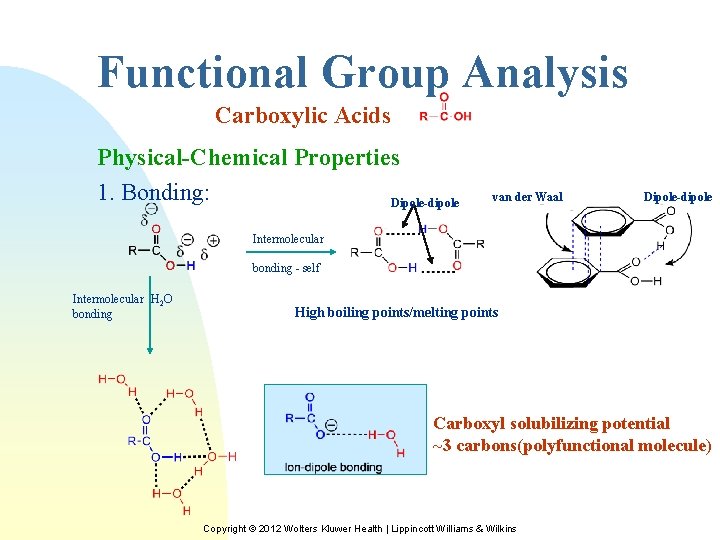
Functional Group Analysis Carboxylic Acids Physical-Chemical Properties 1. Bonding: Dipole-dipole van der Waal Dipole-dipole Intermolecular bonding - self Intermolecular H 2 O bonding High boiling points/melting points Carboxyl solubilizing potential ~3 carbons(polyfunctional molecule) Copyright © 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins

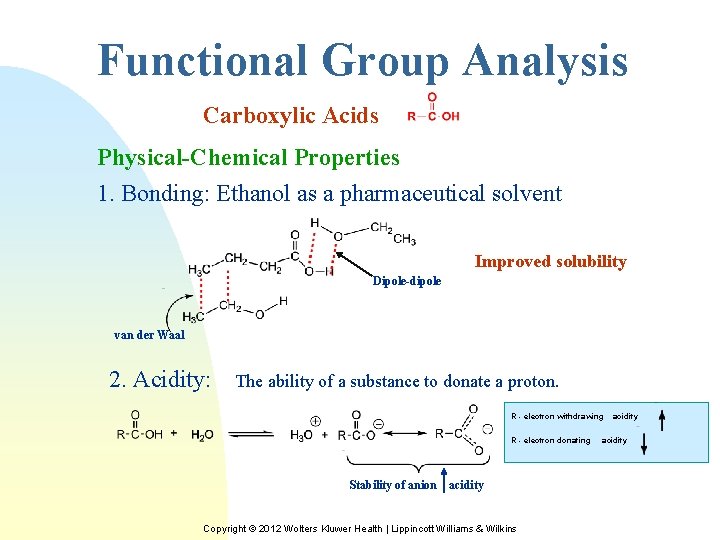
Functional Group Analysis Carboxylic Acids Physical-Chemical Properties 1. Bonding: Ethanol as a pharmaceutical solvent Improved solubility Dipole-dipole van der Waal 2. Acidity: The ability of a substance to donate a proton. R - electron withdrawing R - electron donating Stability of anion acidity Copyright © 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins acidity

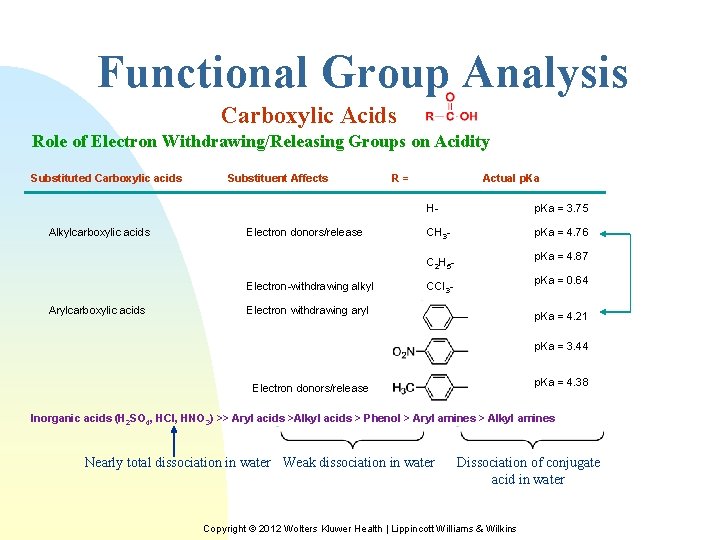
Functional Group Analysis Carboxylic Acids Role of Electron Withdrawing/Releasing Groups on Acidity Substituted Carboxylic acids Alkylcarboxylic acids Substituent Affects Electron donors/release R= Actual p. Ka H- p. Ka = 3. 75 CH 3 - p. Ka = 4. 76 p. Ka = 4. 87 C 2 H 5 Electron-withdrawing alkyl Arylcarboxylic acids p. Ka = 0. 64 CCl 3 - Electron withdrawing aryl p. Ka = 4. 21 p. Ka = 3. 44 p. Ka = 4. 38 Electron donors/release Inorganic acids (H 2 SO 4, HCl, HNO 3) >> Aryl acids >Alkyl acids > Phenol > Aryl amines > Alkyl amines Nearly total dissociation in water Weak dissociation in water Dissociation of conjugate acid in water Copyright © 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins

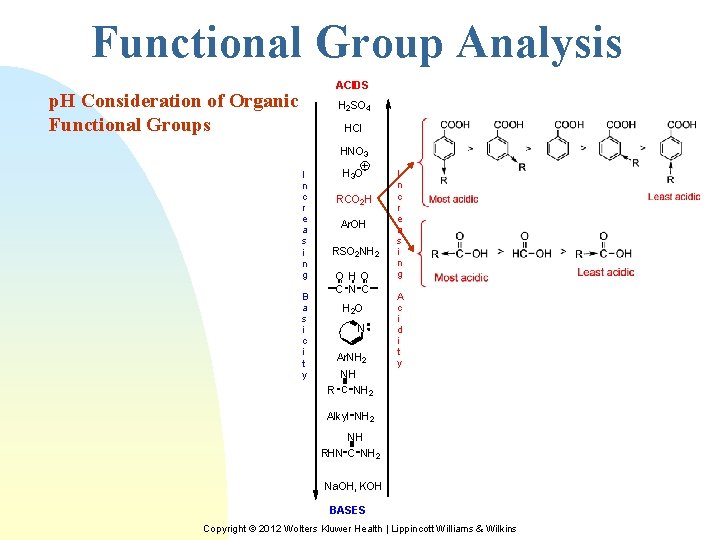
Functional Group Analysis ACIDS p. H Consideration of Organic Functional Groups H 2 SO 4 HCl HNO 3 I n c r e a s i n g B a s i c i t y H 3 O RCO 2 H Ar. OH RSO 2 NH 2 OH O C N C H 2 O N Ar. NH 2 NH R C NH 2 I n c r e a s i n g A c i d i t y Alkyl NH 2 NH RHN C NH 2 Na. OH, KOH BASES Copyright © 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins

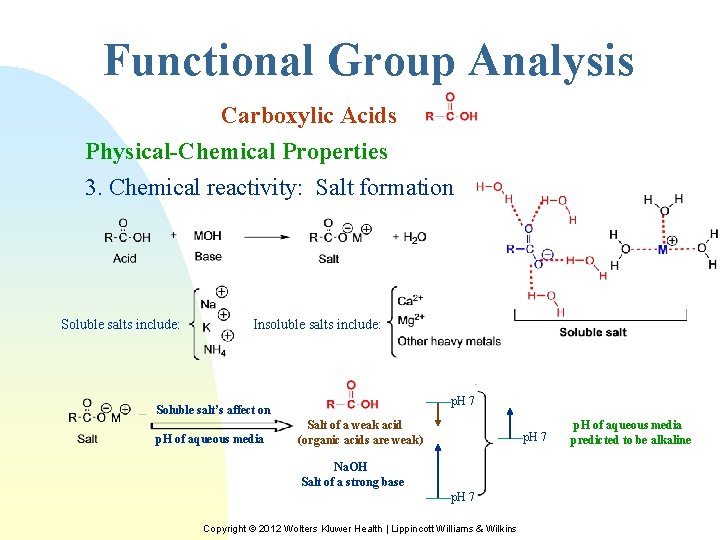
Functional Group Analysis Carboxylic Acids Physical-Chemical Properties 3. Chemical reactivity: Salt formation Soluble salts include: Insoluble salts include: p. H 7 Soluble salt’s affect on p. H of aqueous media Salt of a weak acid (organic acids are weak) p. H 7 Na. OH Salt of a strong base p. H 7 Copyright © 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins p. H of aqueous media predicted to be alkaline

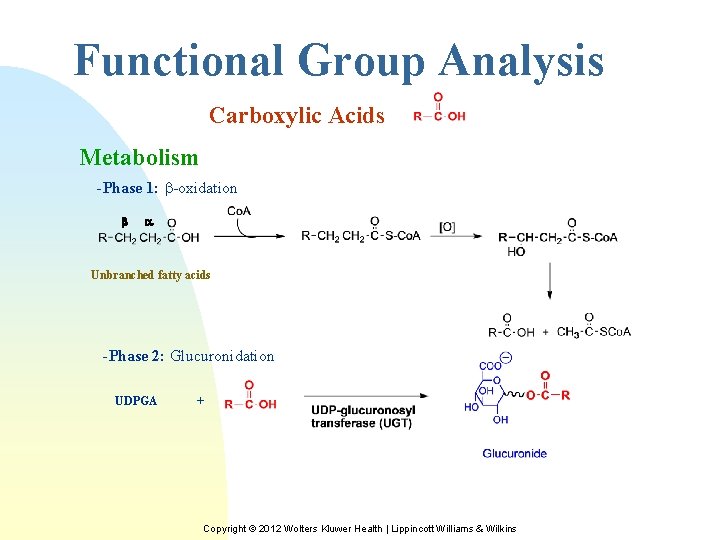
Functional Group Analysis Carboxylic Acids Metabolism -Phase 1: -oxidation b a Unbranched fatty acids -Phase 2: Glucuronidation UDPGA + Copyright © 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins

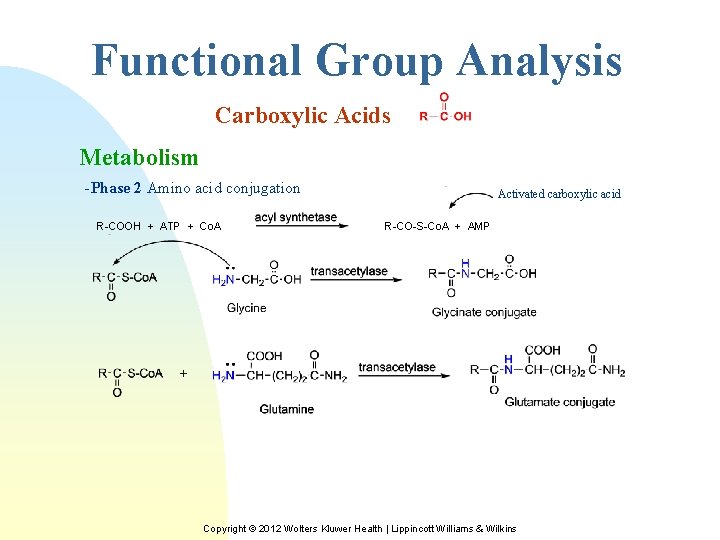
Functional Group Analysis Carboxylic Acids Metabolism -Phase 2 Amino acid conjugation R-COOH + ATP + Co. A Activated carboxylic acid R-CO-S-Co. A + AMP + Copyright © 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins