Review of Microreactors Radmila Jevtic graduate student and




- Slides: 24

Review of Microreactors Radmila Jevtic (graduate student) and Professors Muthanna Al-Dahhan and Milorad P. Dudukovic

Outline n n Background and features Review of gas-liquid microreactors Scale-up methodology Conclusions

Background and Features n n Micro-structured reactor channel diameters: sub mm to mm range Surface/Volume area: 1, 000 -50, 000 m 2/m 3 Sources: 1) A. Günther at al. , Langmuir, 21, 1547 (2005); 2) www. imm-mainz. de/; 3) http: //www. mikroglas. com/

Features: Advantages § High surface-to-volume area; enhanced mass and heat transfer § Laminar flow conditions § Uniform residence time, backmixing minimized (increased precision and accuracy) § High-throughput and use of very small amounts of materials § Low manufacturing, operating, and maintenance costs (if mass produced), and low power consumption § Minimal environmental hazards and increased safety § “Scaling-out” or “numbering-up” instead of scaling-up

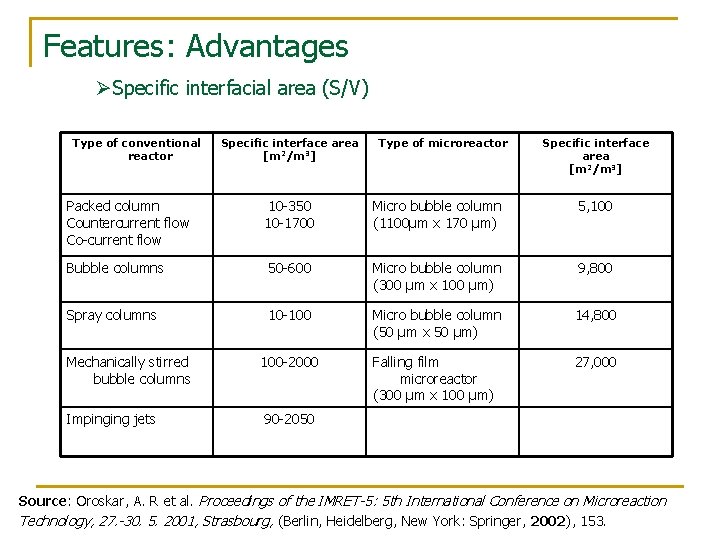
Features: Advantages ØSpecific interfacial area (S/V) Type of conventional reactor Specific interface area [m 2/m 3] Type of microreactor Specific interface area [m 2/m 3] Packed column Countercurrent flow Co-current flow 10 -350 10 -1700 Micro bubble column (1100μm x 170 μm) 5, 100 Bubble columns 50 -600 Micro bubble column (300 μm x 100 μm) 9, 800 Spray columns 10 -100 Micro bubble column (50 μm x 50 μm) 14, 800 Mechanically stirred bubble columns 100 -2000 Falling film microreactor (300 μm x 100 μm) 27, 000 Impinging jets 90 -2050 Source: Oroskar, A. R et al. Proceedings of the IMRET-5: 5 th International Conference on Microreaction Technology, 27. -30. 5. 2001, Strasbourg, (Berlin, Heidelberg, New York: Springer, 2002), 153.

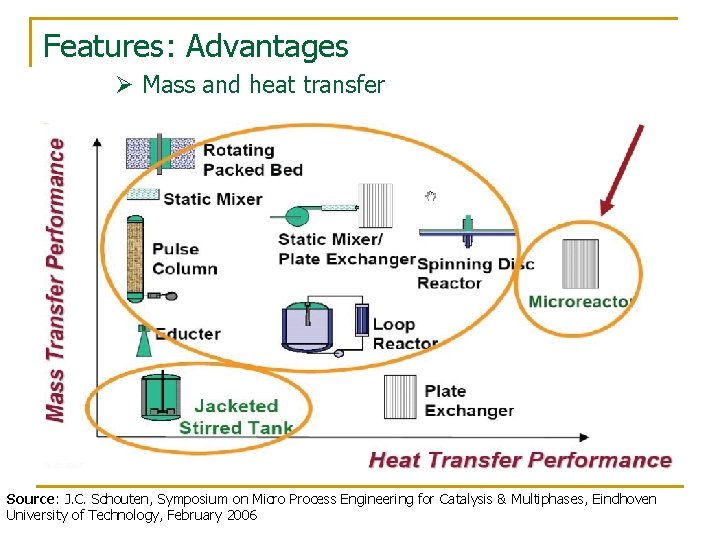
Features: Advantages Ø Mass and heat transfer Source: J. C. Schouten, Symposium on Micro Process Engineering for Catalysis & Multiphases, Eindhoven University of Technology, February 2006

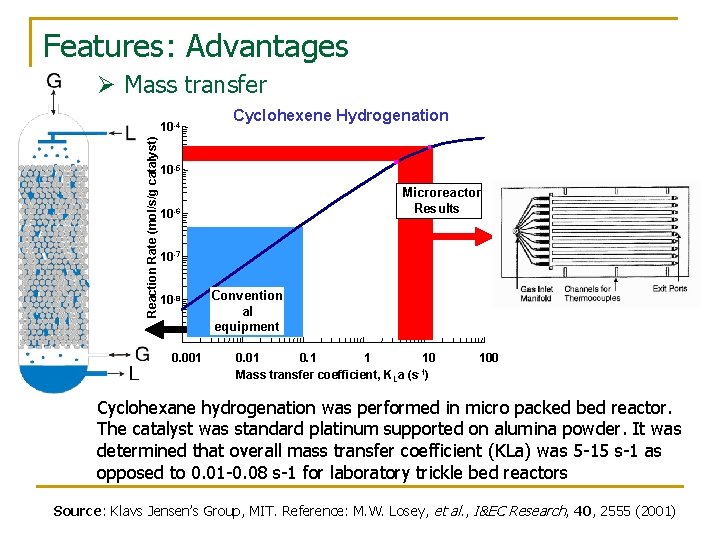
Features: Advantages Ø Mass transfer Reaction Rate (mol/s/g catalyst) 10 -4 Cyclohexene Hydrogenation 10 -5 Microreactor Results 10 -6 10 -7 10 -8 0. 001 Convention al equipment 0. 01 0. 1 1 10 -1 Mass transfer coefficient, KLa (s ) 100 Cyclohexane hydrogenation was performed in micro packed bed reactor. The catalyst was standard platinum supported on alumina powder. It was determined that overall mass transfer coefficient (KLa) was 5 -15 s-1 as opposed to 0. 01 -0. 08 s-1 for laboratory trickle bed reactors Source: Klavs Jensen’s Group, MIT. Reference: M. W. Losey, et al. , I&EC Research, 40, 2555 (2001)

Features: Drawbacks § Fast reaction rates are required due to the short § § § residence times Fouling and clogging Maintenance, ageing, regeneration Lack of experience with commercial processes Catalyst deactivation, life on stream unknown Only few technology providers

Potential application for microreactors n n Synthesis of hazardous gases: chlorine, iso-cyanates, hydrogen cyanide, phosgene… Hydrogen production via steam reforming, partial oxidation of methane, from higher alkanes and alcohol to syngas Synthesis of ethylene oxide, propylene to acrolein, oxidative dehydrogenation of alcohols to aldehydes Oxidation of ammonia Source: Dupont et al , Minisymposium on Micro Process Engineering for Catalysis & Multiphases, Eindhoven University of Technology, February 2006

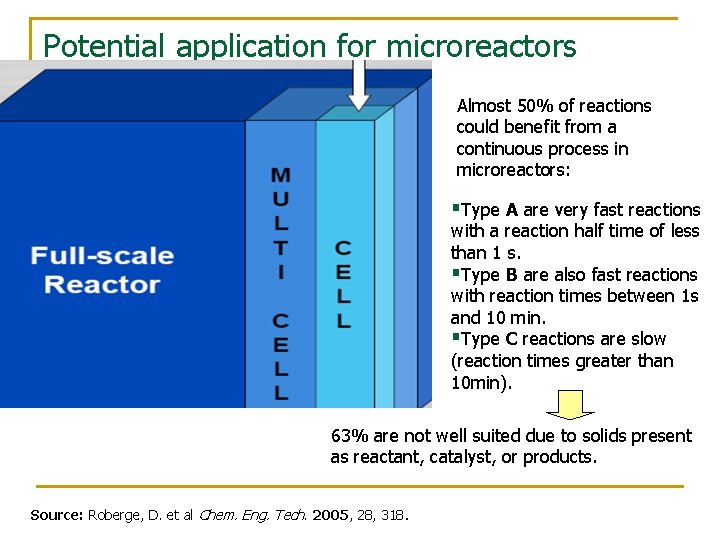
Potential application for microreactors Almost 50% of reactions could benefit from a continuous process in microreactors: §Type A are very fast reactions with a reaction half time of less than 1 s. §Type B are also fast reactions with reaction times between 1 s and 10 min. §Type C reactions are slow (reaction times greater than 10 min). 63% are not well suited due to solids present as reactant, catalyst, or products. Source: Roberge, D. et al Chem. Eng. Tech. 2005, 28, 318.

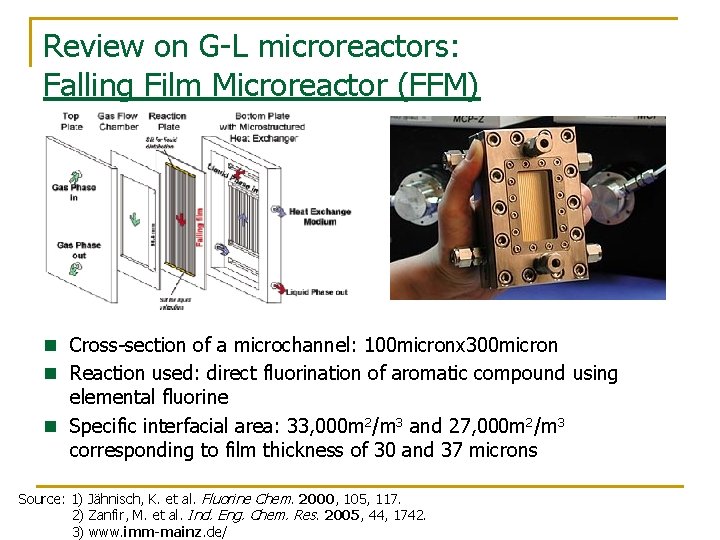
Review on G-L microreactors: Falling Film Microreactor (FFM) n Cross-section of a microchannel: 100 micronx 300 micron n Reaction used: direct fluorination of aromatic compound using elemental fluorine n Specific interfacial area: 33, 000 m 2/m 3 and 27, 000 m 2/m 3 corresponding to film thickness of 30 and 37 microns Source: 1) Jähnisch, K. et al. Fluorine Chem. 2000, 105, 117. 2) Zanfir, M. et al. Ind. Eng. Chem. Res. 2005, 44, 1742. 3) www. imm-mainz. de/

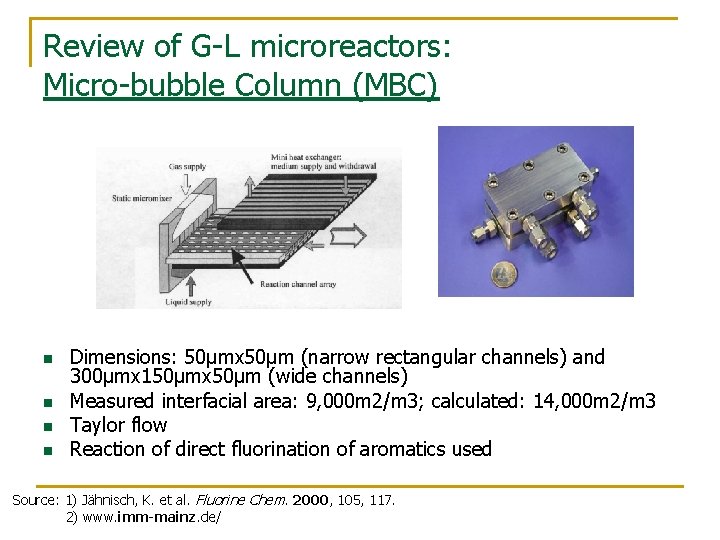
Review of G-L microreactors: Micro-bubble Column (MBC) n n Dimensions: 50μmx 50μm (narrow rectangular channels) and 300μmx 150μmx 50μm (wide channels) Measured interfacial area: 9, 000 m 2/m 3; calculated: 14, 000 m 2/m 3 Taylor flow Reaction of direct fluorination of aromatics used Source: 1) Jähnisch, K. et al. Fluorine Chem. 2000, 105, 117. 2) www. imm-mainz. de/

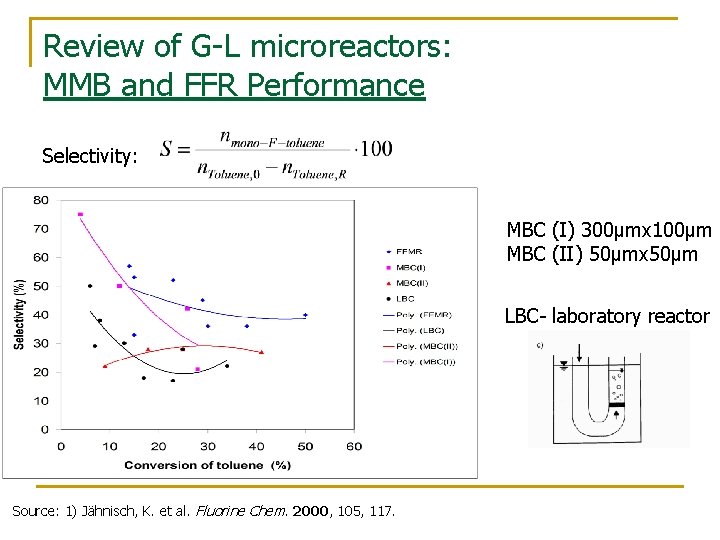
Review of G-L microreactors: MMB and FFR Performance Selectivity: MBC (I) 300μmx 100μm MBC (II) 50μmx 50μm LBC- laboratory reactor Source: 1) Jähnisch, K. et al. Fluorine Chem. 2000, 105, 117.

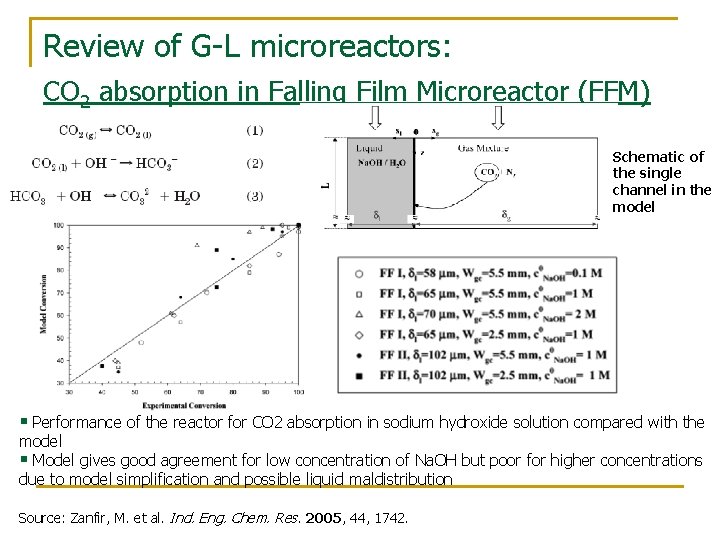
Review of G-L microreactors: CO 2 absorption in Falling Film Microreactor (FFM) Schematic of the single channel in the model § Performance of the reactor for CO 2 absorption in sodium hydroxide solution compared with the model § Model gives good agreement for low concentration of Na. OH but poor for higher concentrations due to model simplification and possible liquid maldistribution Source: Zanfir, M. et al. Ind. Eng. Chem. Res. 2005, 44, 1742.

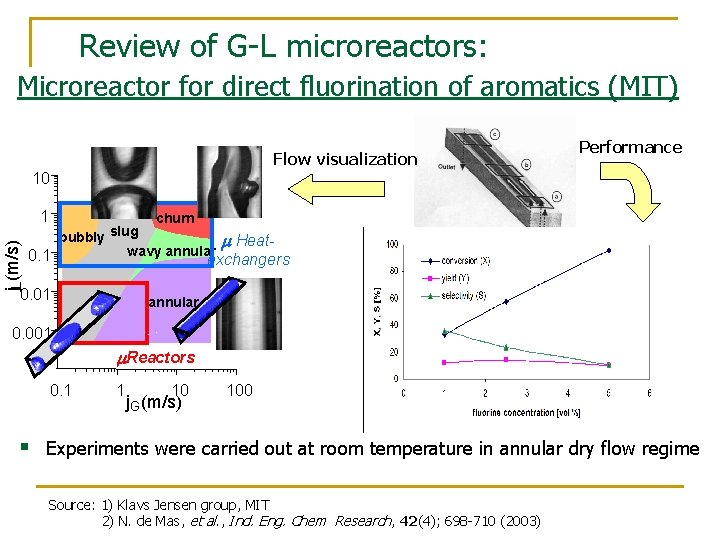
Review of G-L microreactors: Microreactor for direct fluorination of aromatics (MIT) Flow visualization Performance 10 j. L(m/s) Slug 1 churn slug bubbly m Heatwavy annular 0. 1 exchangers 0. 01 annular Annular 0. 001 m. Reactors 0. 1 1 10 j. G (m/s) 100 § Experiments were carried out at room temperature in annular dry flow regime Source: 1) Klavs Jensen group, MIT 2) N. de Mas, et al. , Ind. Eng. Chem Research, 42(4); 698 -710 (2003)

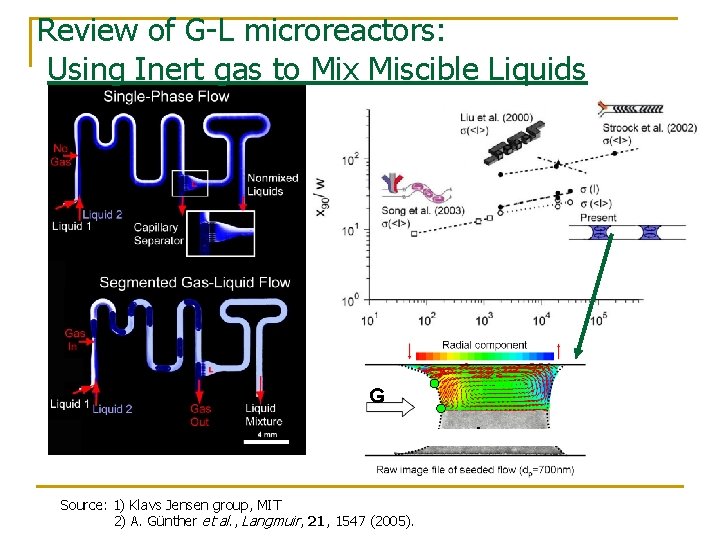
Review of G-L microreactors: Using Inert gas to Mix Miscible Liquids G L Source: 1) Klavs Jensen group, MIT 2) A. Günther et al. , Langmuir, 21, 1547 (2005).

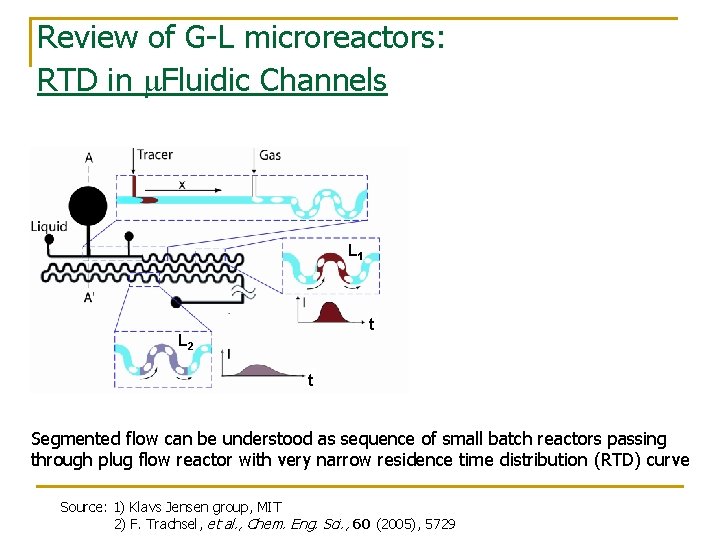
Review of G-L microreactors: RTD in m. Fluidic Channels L 1 t L 2 t Segmented flow can be understood as sequence of small batch reactors passing through plug flow reactor with very narrow residence time distribution (RTD) curve Source: 1) Klavs Jensen group, MIT 2) F. Trachsel, et al. , Chem. Eng. Sci. , 60 (2005), 5729

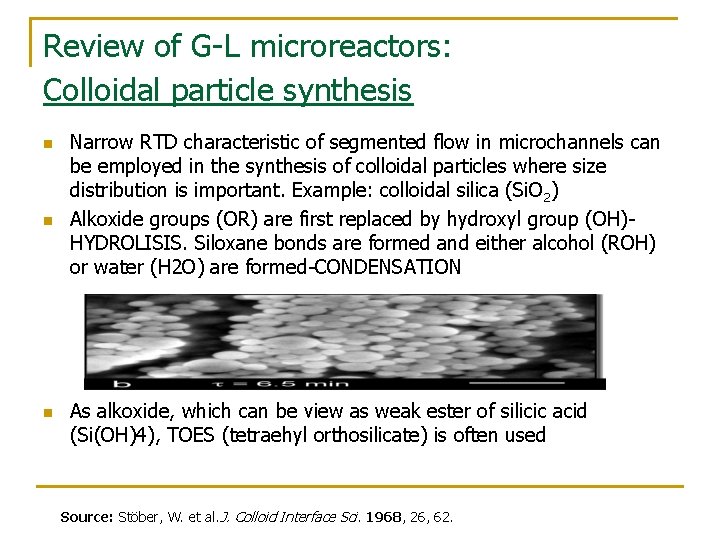
Review of G-L microreactors: Colloidal particle synthesis n n n Narrow RTD characteristic of segmented flow in microchannels can be employed in the synthesis of colloidal particles where size distribution is important. Example: colloidal silica (Si. O 2) Alkoxide groups (OR) are first replaced by hydroxyl group (OH)HYDROLISIS. Siloxane bonds are formed and either alcohol (ROH) or water (H 2 O) are formed-CONDENSATION As alkoxide, which can be view as weak ester of silicic acid (Si(OH)4), TOES (tetraehyl orthosilicate) is often used Source: Stöber, W. et al. J. Colloid Interface Sci. 1968, 26, 62.

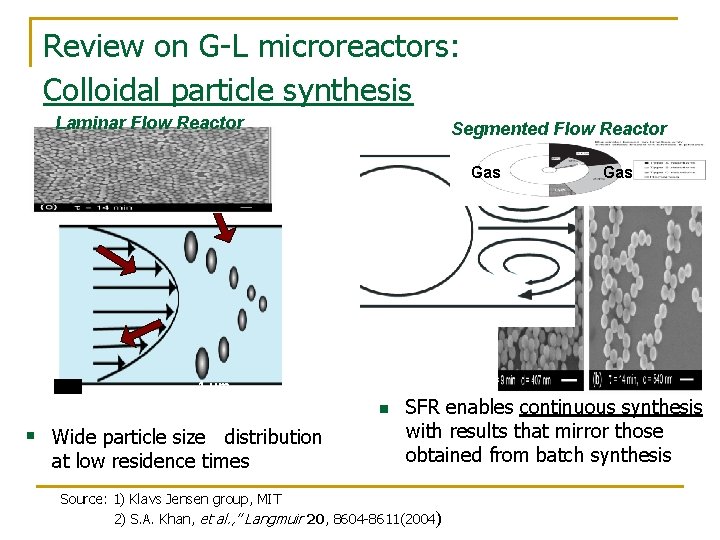
Review on G-L microreactors: Colloidal particle synthesis Laminar Flow Reactor Segmented Flow Reactor Gas 1 µm n § Wide particle size distribution at low residence times Gas SFR enables continuous synthesis 1 µm with results that mirror those obtained from batch synthesis Source: 1) Klavs Jensen group, MIT 2) S. A. Khan, et al. , ” Langmuir 20, 8604 -8611(2004)

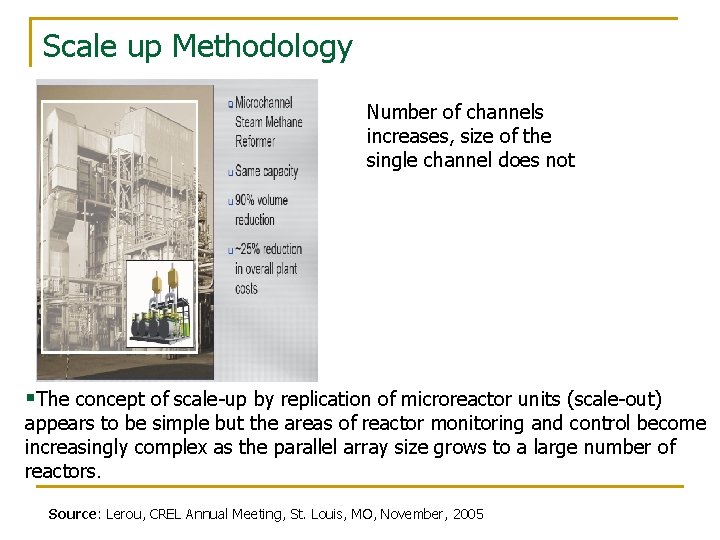
Scale up Methodology Number of channels increases, size of the single channel does not §The concept of scale-up by replication of microreactor units (scale-out) appears to be simple but the areas of reactor monitoring and control become increasingly complex as the parallel array size grows to a large number of reactors. Source: Lerou, CREL Annual Meeting, St. Louis, MO, November, 2005

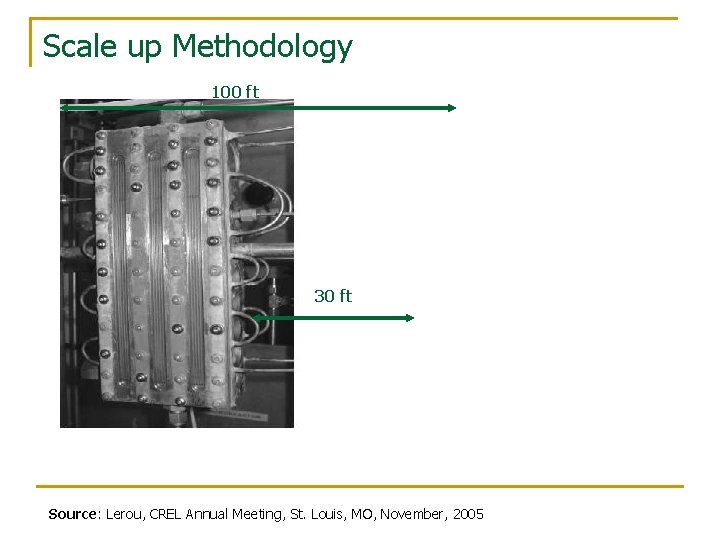
Scale up Methodology 100 ft 30 ft Source: Lerou, CREL Annual Meeting, St. Louis, MO, November, 2005

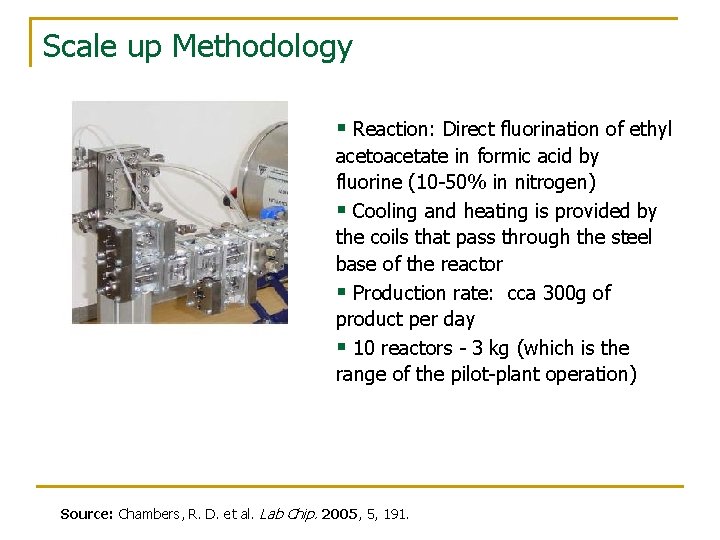
Scale up Methodology § Reaction: Direct fluorination of ethyl acetoacetate in formic acid by fluorine (10 -50% in nitrogen) § Cooling and heating is provided by the coils that pass through the steel base of the reactor § Production rate: cca 300 g of product per day § 10 reactors - 3 kg (which is the range of the pilot-plant operation) Source: Chambers, R. D. et al. Lab Chip. 2005, 5, 191.

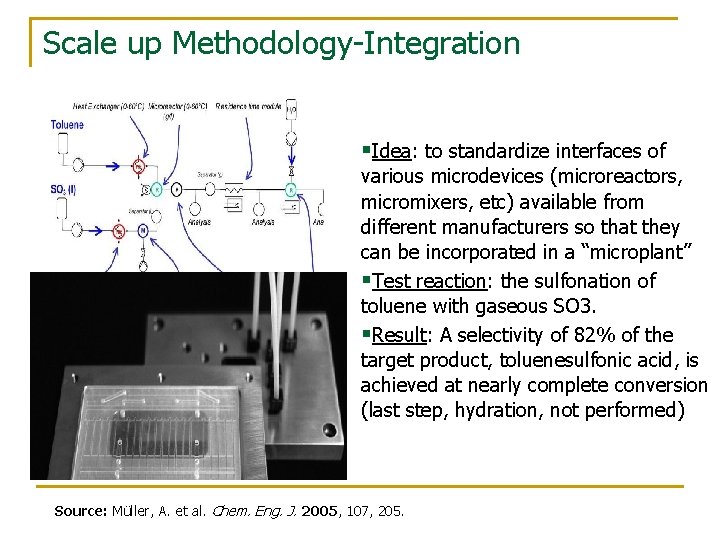
Scale up Methodology-Integration §Idea: to standardize interfaces of various microdevices (microreactors, micromixers, etc) available from different manufacturers so that they can be incorporated in a “microplant” §Test reaction: the sulfonation of toluene with gaseous SO 3. §Result: A selectivity of 82% of the target product, toluenesulfonic acid, is achieved at nearly complete conversion (last step, hydration, not performed) Source: Müller, A. et al. Chem. Eng. J. 2005, 107, 205.

Conclusions n à à à Promising technology: enhanced mass and heat transfer efficient process intensification inherently safe operation uniform residence time Some issues still remain: integration with sensors, actuators, and other associated equipment, such as pumps; reactor monitoring and control; high activity stable catalysts needed.