Review of Carbon Classification Type of C Attached
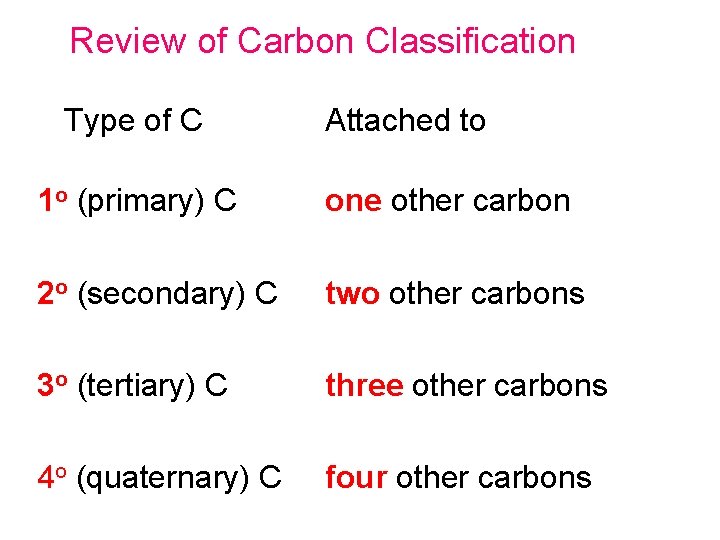
Review of Carbon Classification Type of C Attached to 1 o (primary) C one other carbon 2 o (secondary) C two other carbons 3 o (tertiary) C three other carbons 4 o (quaternary) C four other carbons
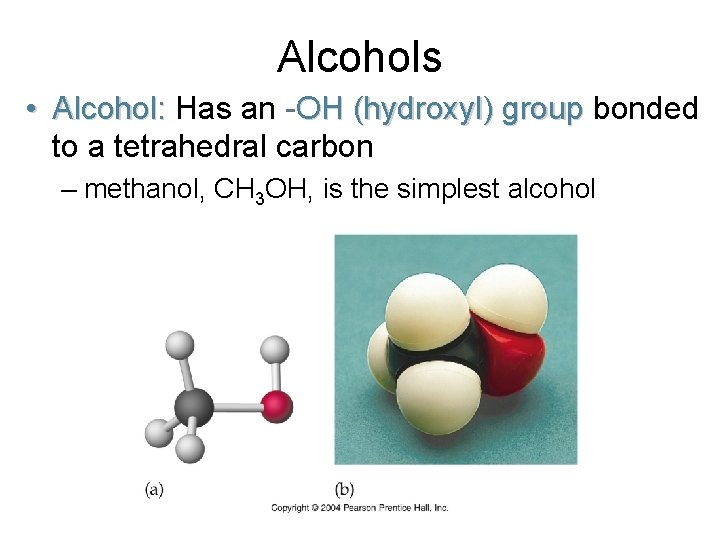
Alcohols • Alcohol: Has an -OH (hydroxyl) group bonded to a tetrahedral carbon – methanol, CH 3 OH, is the simplest alcohol
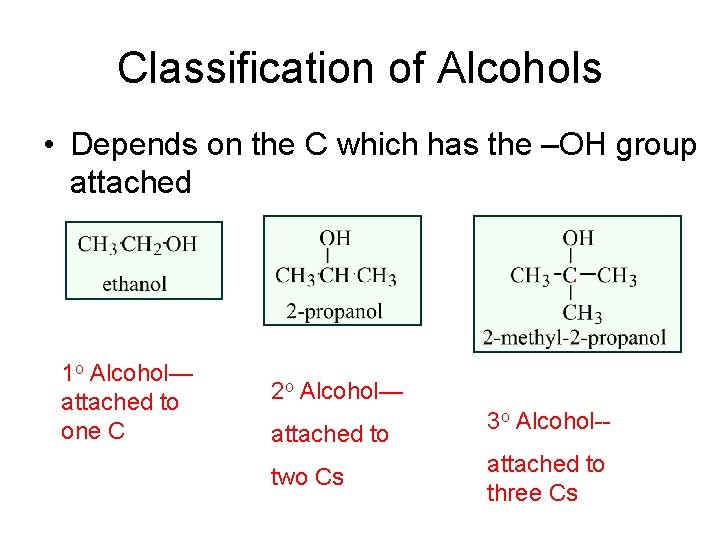
Classification of Alcohols • Depends on the C which has the –OH group attached 1 o Alcohol— attached to one C 2 o Alcohol— attached to two Cs 3 o Alcohol-attached to three Cs

Alcohol Nomenclature 1. Find longest carbon chain that contains the -OH group (parent chain) 2. Number chain from end that gives the -OH the lower number 3. Change the ending -e to -ol 4. Use a number to show the location of the -OH group For cyclic alcohols, the carbon with the -OH group is C-1 5. Name and number substituents and list them in alphabetical order
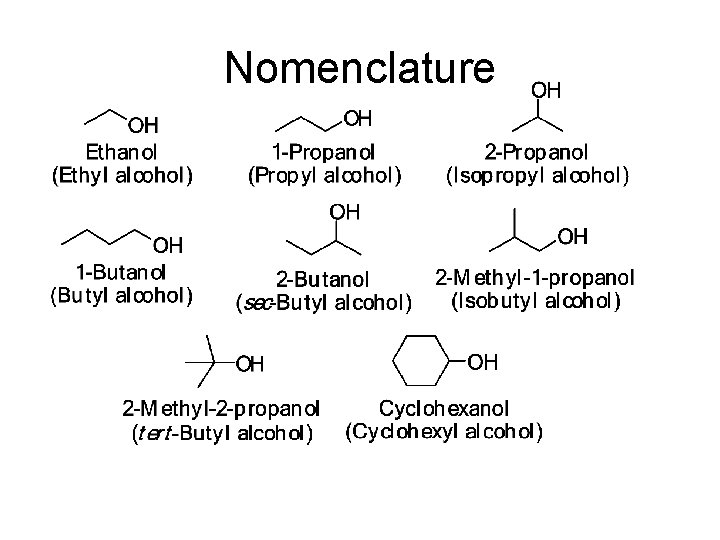
Nomenclature
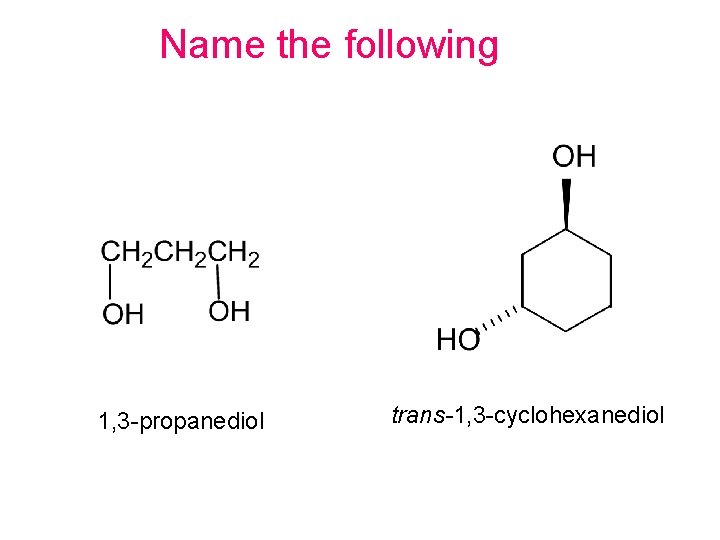
Name the following 1, 3 -propanediol trans-1, 3 -cyclohexanediol
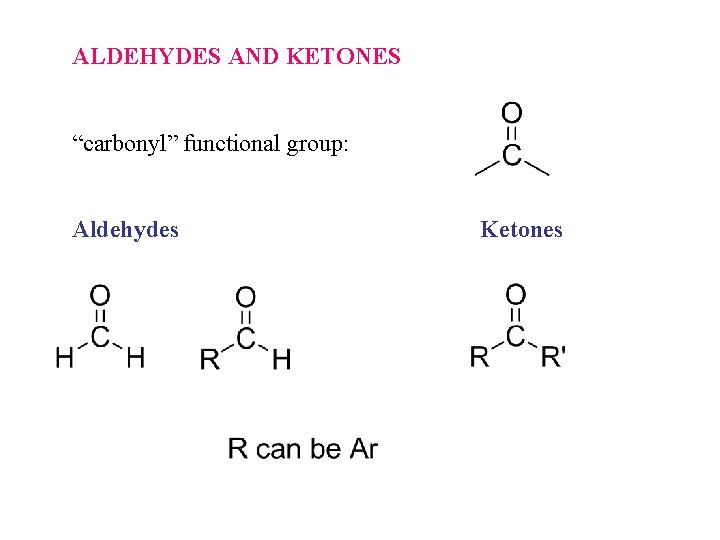
ALDEHYDES AND KETONES “carbonyl” functional group: Aldehydes Ketones
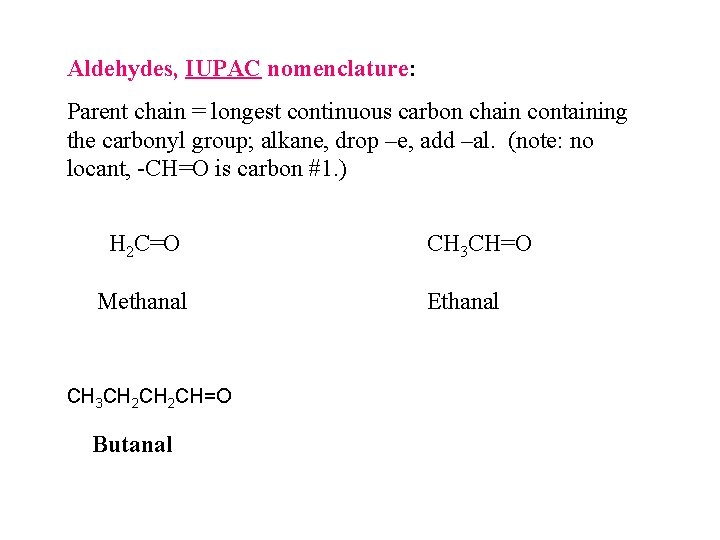
Aldehydes, IUPAC nomenclature: Parent chain = longest continuous carbon chain containing the carbonyl group; alkane, drop –e, add –al. (note: no locant, -CH=O is carbon #1. ) H 2 C=O Methanal CH 3 CH 2 CH=O Butanal CH 3 CH=O Ethanal
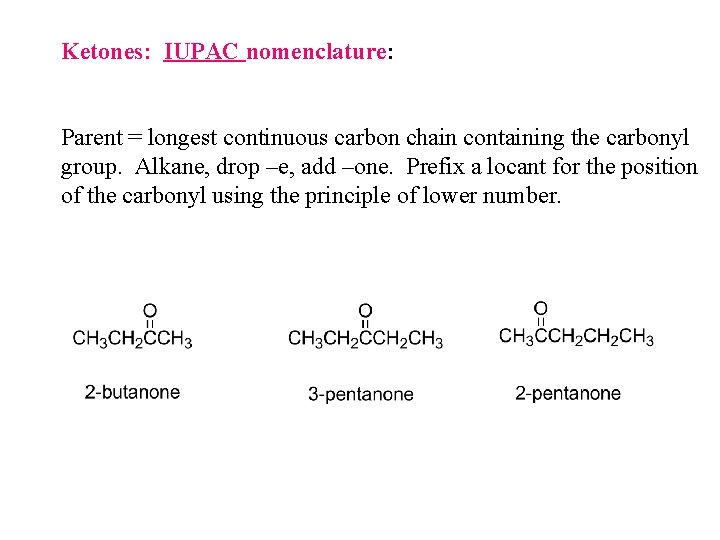
Ketones: IUPAC nomenclature: Parent = longest continuous carbon chain containing the carbonyl group. Alkane, drop –e, add –one. Prefix a locant for the position of the carbonyl using the principle of lower number.
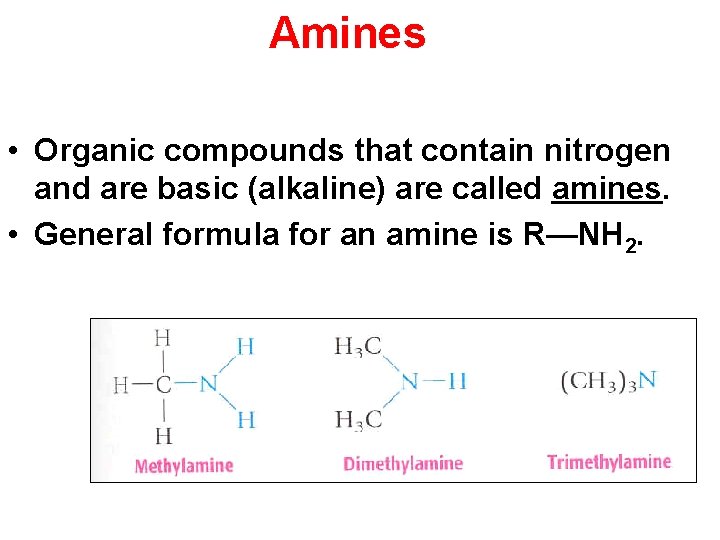
Amines • Organic compounds that contain nitrogen and are basic (alkaline) are called amines. • General formula for an amine is R—NH 2. Section 14. 4
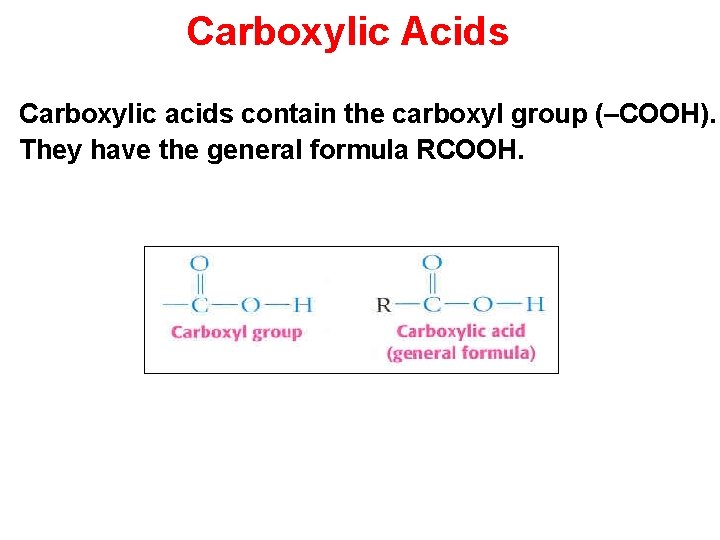
Carboxylic Acids Carboxylic acids contain the carboxyl group (–COOH). They have the general formula RCOOH. Section 14. 4
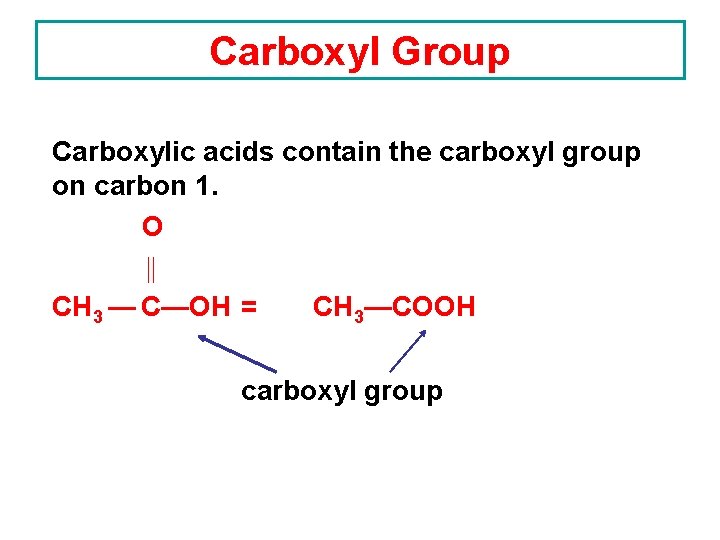
Carboxyl Group Carboxylic acids contain the carboxyl group on carbon 1. O CH 3 — C—OH = CH 3—COOH carboxyl group
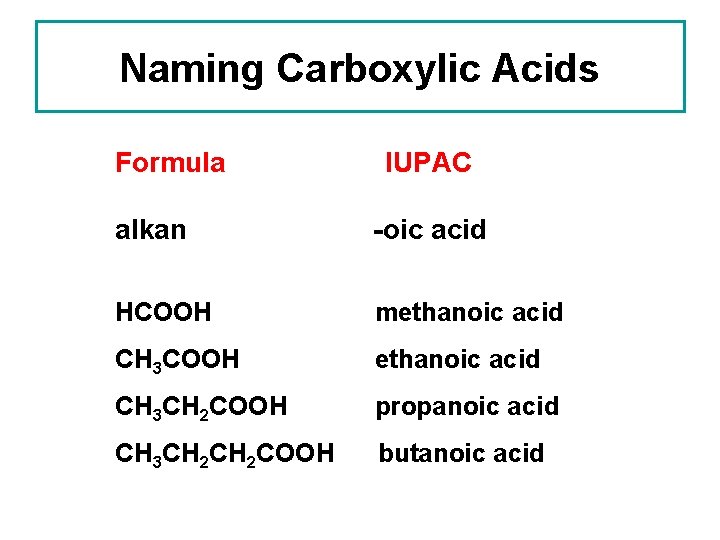
Naming Carboxylic Acids Formula IUPAC alkan -oic acid HCOOH methanoic acid CH 3 COOH ethanoic acid CH 3 CH 2 COOH propanoic acid CH 3 CH 2 COOH butanoic acid
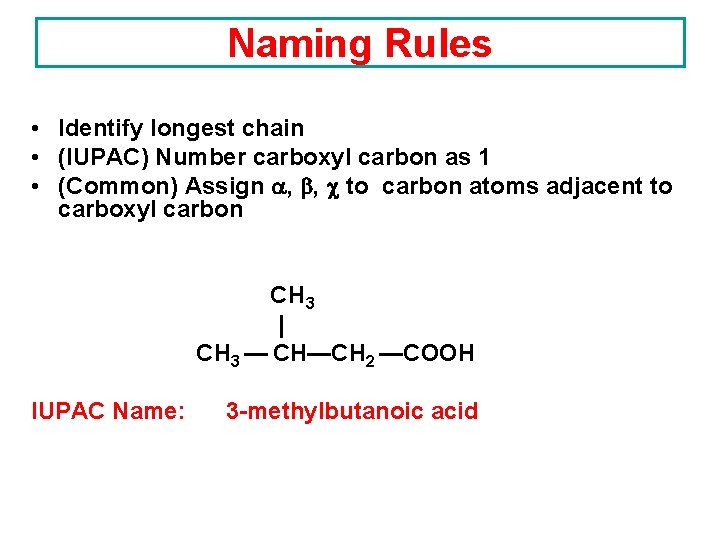
Naming Rules • Identify longest chain • (IUPAC) Number carboxyl carbon as 1 • (Common) Assign , , to carbon atoms adjacent to carboxyl carbon CH 3 | CH 3 — CH—CH 2 —COOH IUPAC Name: 3 -methylbutanoic acid
- Slides: 14