Review of Basic Chemistry A Subatomic Particles Atoms

Review of Basic Chemistry
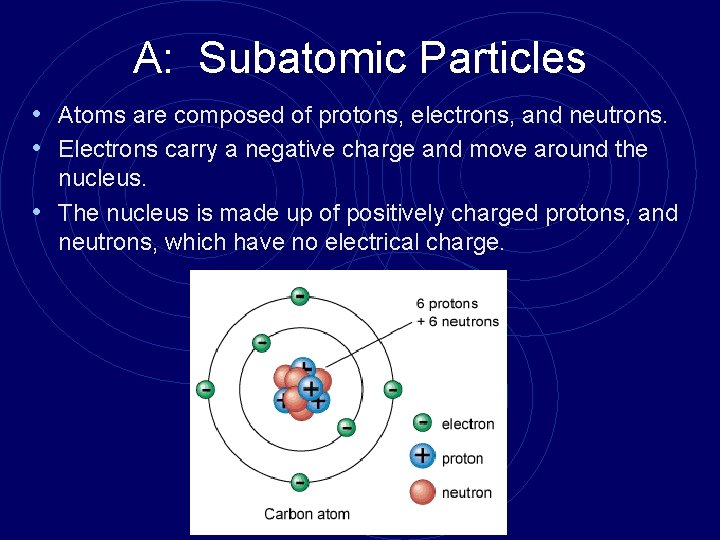
A: Subatomic Particles • Atoms are composed of protons, electrons, and neutrons. • Electrons carry a negative charge and move around the nucleus. • The nucleus is made up of positively charged protons, and neutrons, which have no electrical charge.
name proton electron neutron charge symbol mass location
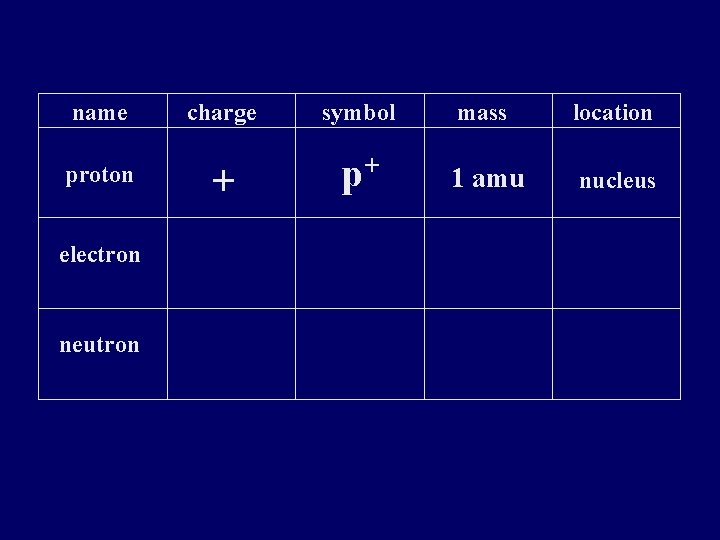
name proton electron neutron charge symbol mass location + p+ 1 amu nucleus
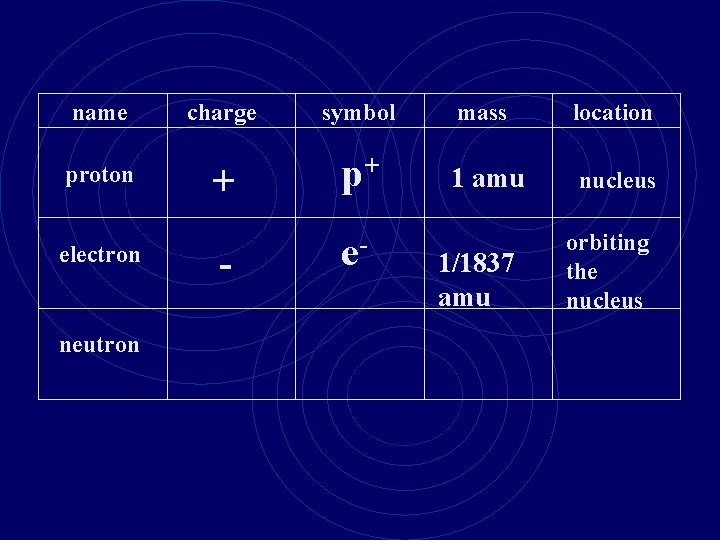
name proton electron neutron charge symbol mass location + p+ 1 amu nucleus - e- 1/1837 amu orbiting the nucleus
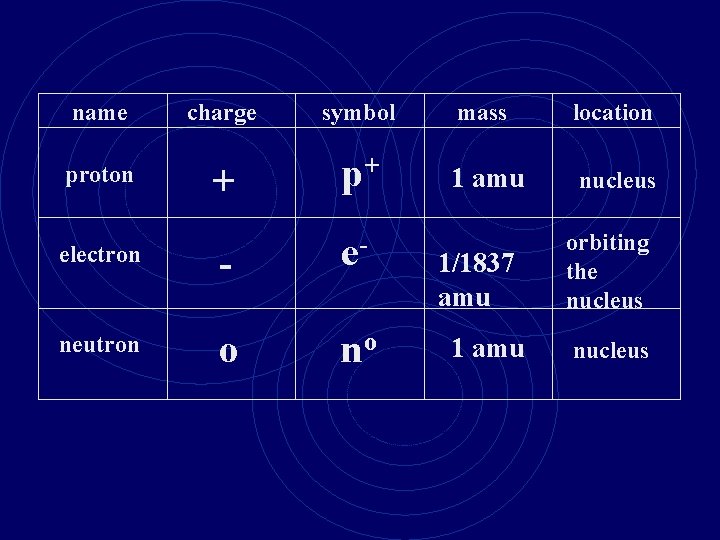
name charge symbol mass location + p+ 1 amu nucleus electron - e- neutron o no proton 1/1837 amu 1 amu orbiting the nucleus
B: Structure of Atoms • In an atom, the number of protons = the number of electrons. • The mass number = protons + neutrons. • Each electron orbital can hold a certain number of electrons: 1 st orbital can hold only 2 e- (inner most) 2 nd orbital can hold only 8 e 3 rd orbital can hold only 8 e 4 th orbital can hold only 18 e-
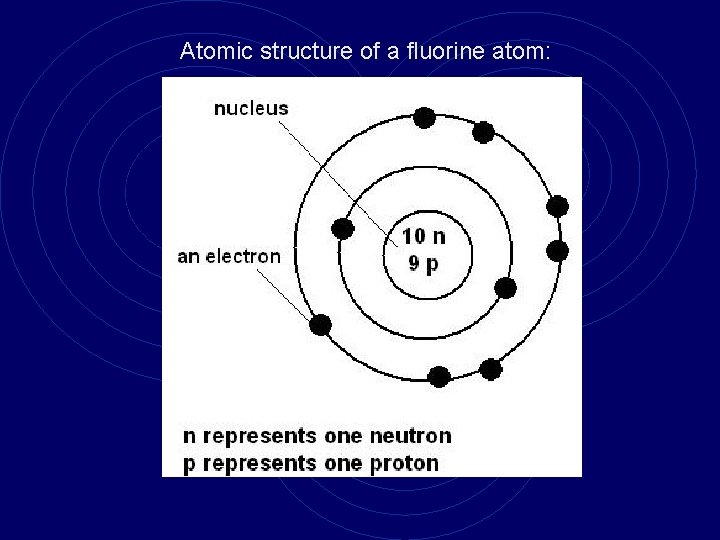
Atomic structure of a fluorine atom:
The Periodic Table • The periodic table is made of three types of elements; 1. metals (left side) 2. non-metals (right side) 3. metalloids (middle) boron, silicon, germanium, arsenic, antimony, tellurium, polonium

A chemical group/family is a vertical column of elements that have similar physical and chemical properties. On the periodic table there are 18 vertical groups. Column 1: alkali metals Column 2: alkaline earth metals Column 3 -11: transition metals Column 17: halogens Column 18: noble gases/inert gases Two Rows at Bottom: rare earth metals

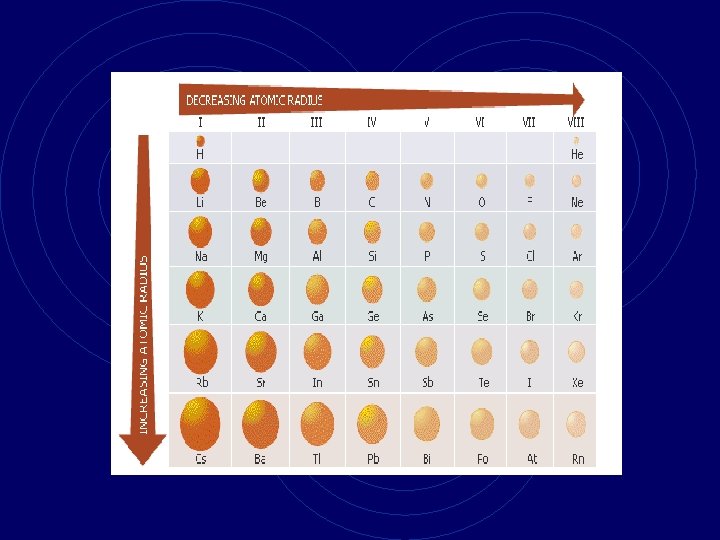
Periods • Periods are horizontal rows of elements. • The first period (row) contains 2 elements. • The second period (row) contains 8 elements etc. • The properties of an element gradually change across a period. • The size of an atom gets smaller as you move across a period.
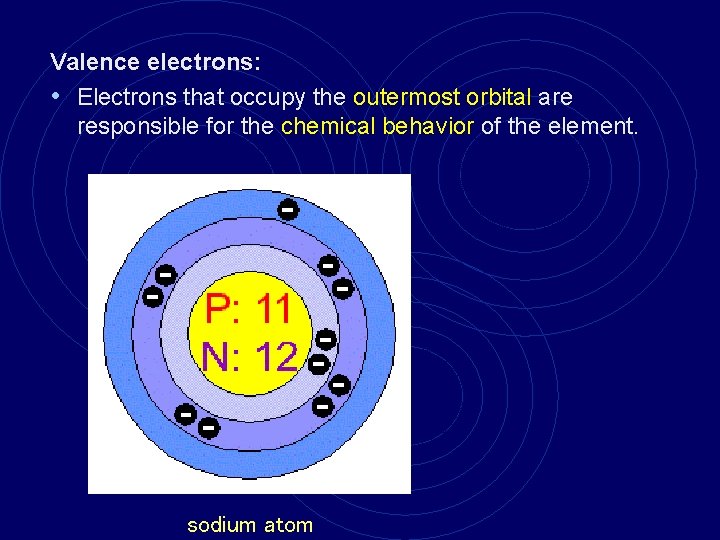
Valence electrons: • Electrons that occupy the outermost orbital are responsible for the chemical behavior of the element. sodium atom
- Slides: 13