REVIEW How many particles are present in one














- Slides: 26

REVIEW

How many particles are present in one mole of particles?

If each image represents one mole of the substance pictured, which contains the greatest amount of substance?

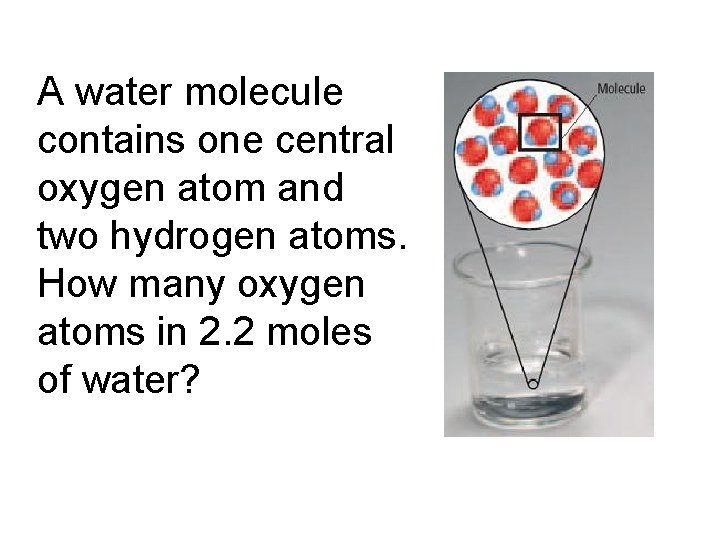
A water molecule contains one central oxygen atom and two hydrogen atoms. How many oxygen atoms in 2. 2 moles of water?

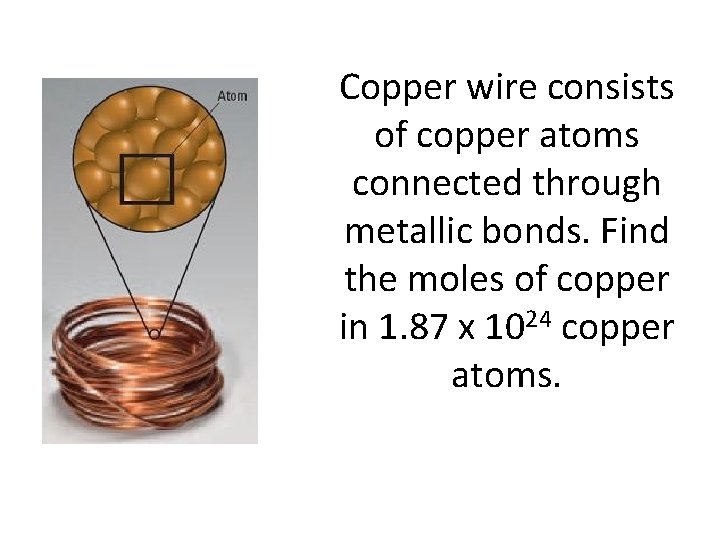
Copper wire consists of copper atoms connected through metallic bonds. Find the moles of copper in 1. 87 x 1024 copper atoms.

Mass and The Mole

Medium, Large, and Extra Large • The size of the egg does not affect how many come in a carton.

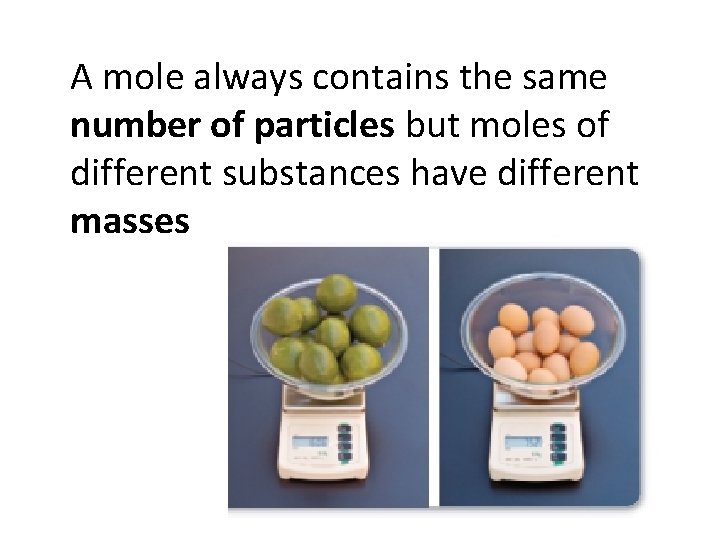
A mole always contains the same number of particles but moles of different substances have different masses

Molar mass • mass in grams of one mole of any pure substance

Element’s molar mass = its atomic mass • Measured in units g/mol Carbon has an atomic mass of 12. 011 so the molar mass of Carbon is 12. 011 g/mol and 1 mole of Carbon has a mass of 12. 011 g

What is the molar mass of… • He • W • Cu • Na. Cl • H₂O₂

Using Molar Mass • You are selling jelly beans by the dozen at a candy sale. It takes too long to count out each dozen, so you decide to measure the jelly beans by mass. 1 dozen jelly beans = 35 g What should the mass be for 5 dozen jelly beans?

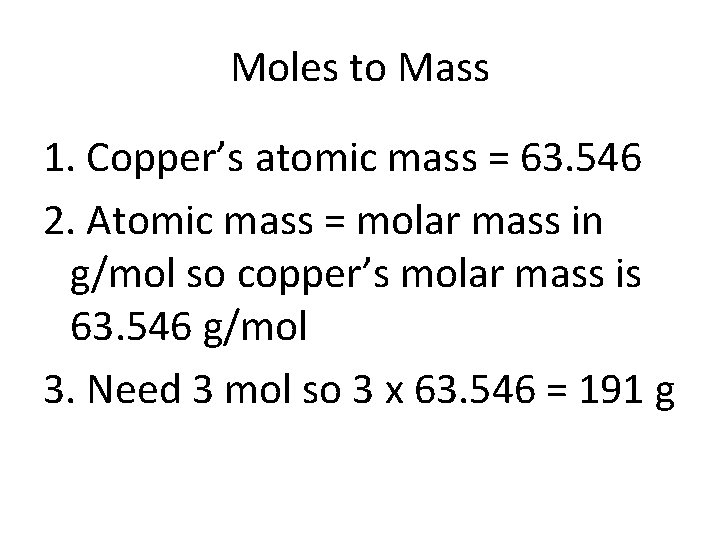
Moles to Mass • In a chemistry lab, you need 3. 00 mol of Copper for a chemical reaction. How do you measure that amount?

Moles to Mass 1. Find the atomic mass for the substance 2. Atomic mass = molar mass in g/mol 3. Multiply the molar mass by number of mol needed

Moles to Mass 1. Copper’s atomic mass = 63. 546 2. Atomic mass = molar mass in g/mol so copper’s molar mass is 63. 546 g/mol 3. Need 3 mol so 3 x 63. 546 = 191 g

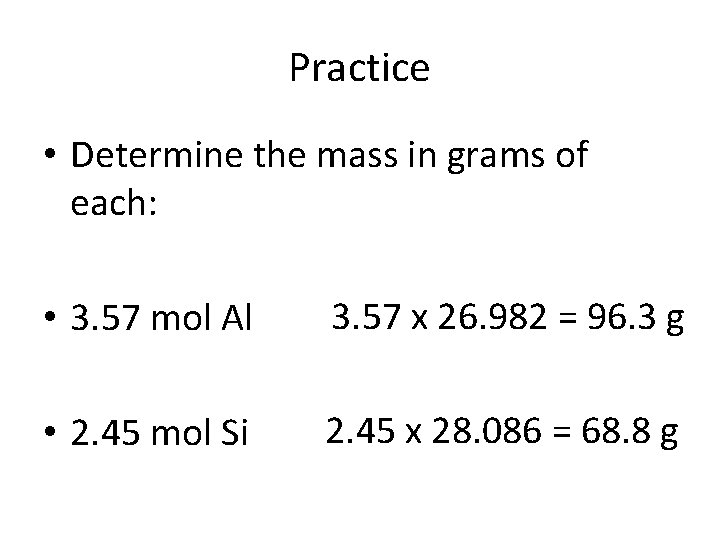
Practice • Determine the mass in grams of each: • 3. 57 mol Al 3. 57 x 26. 982 = 96. 3 g • 2. 45 mol Si 2. 45 x 28. 086 = 68. 8 g

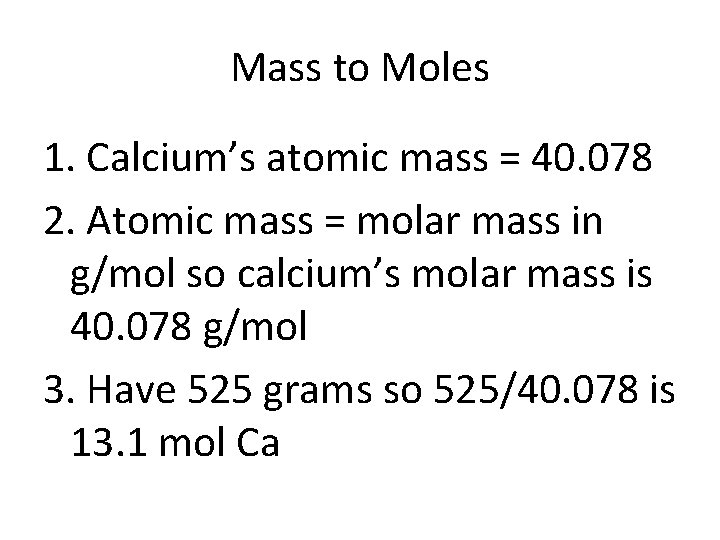
Mass to Moles • How many moles of Calcium are in 525 g Ca?

Mass to Moles 1. Find the atomic mass for the substance 2. Atomic mass = molar mass in g/mol 3. Divide total mass by the molar mass

Mass to Moles 1. Calcium’s atomic mass = 40. 078 2. Atomic mass = molar mass in g/mol so calcium’s molar mass is 40. 078 g/mol 3. Have 525 grams so 525/40. 078 is 13. 1 mol Ca

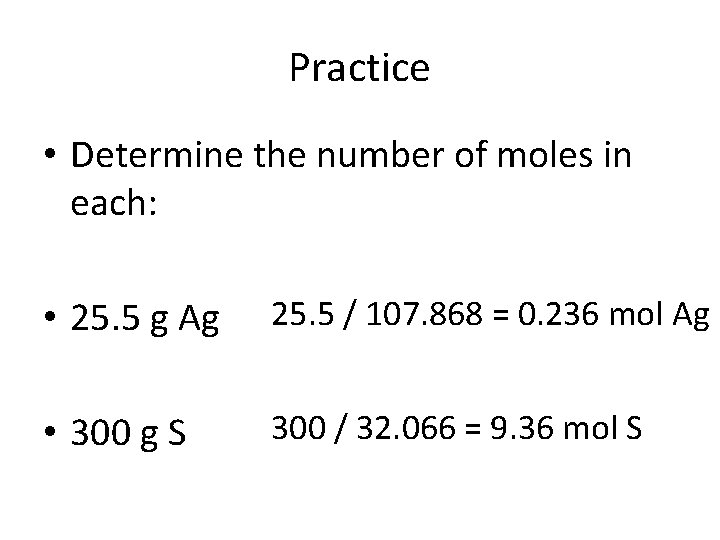
Practice • Determine the number of moles in each: • 25. 5 g Ag 25. 5 / 107. 868 = 0. 236 mol Ag • 300 g S 300 / 32. 066 = 9. 36 mol S

Converting Mass to # of Particles
Mass to # of Particles • You have 550 g of jelly beans left unsold. Without counting, how do you figure out how many jelly beans that is? 35 g = 1 dozen = 12

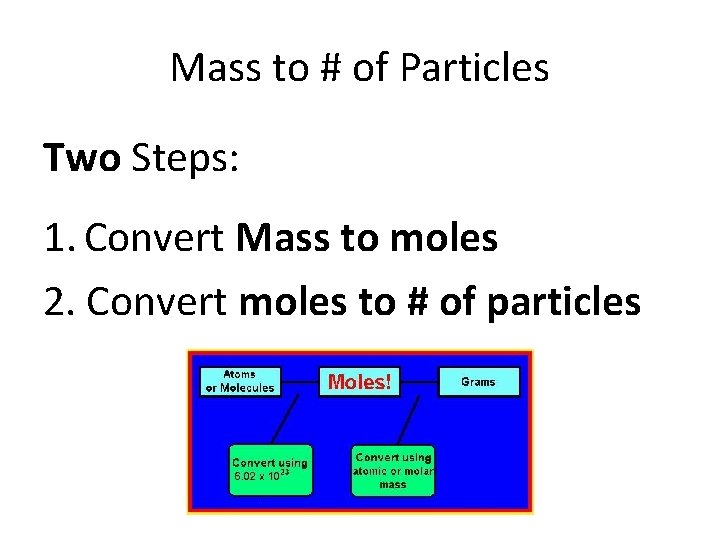
Mass to # of Particles Two Steps: 1. Convert Mass to moles 2. Convert moles to # of particles
How many atoms are in a gold coin with a mass of 31. 1 g? • Mass to moles • Moles to # of particles

If a neon bulb contains 2. 69 x 10²² atoms, what is the mass in grams? • # of particles to Moles • Moles to Mass

Mass 2. 5 g Au Moles 0. 0127 mol # of Particles 7. 64 x 10²¹ 4. 95 mol Zn 3. 95 x 10²³ atoms C 12. 5 mol Cu 8. 75 x 10²¹ atoms Sc 49. 6 g Se