Review Alkanes Cn H 2 n2 butane C
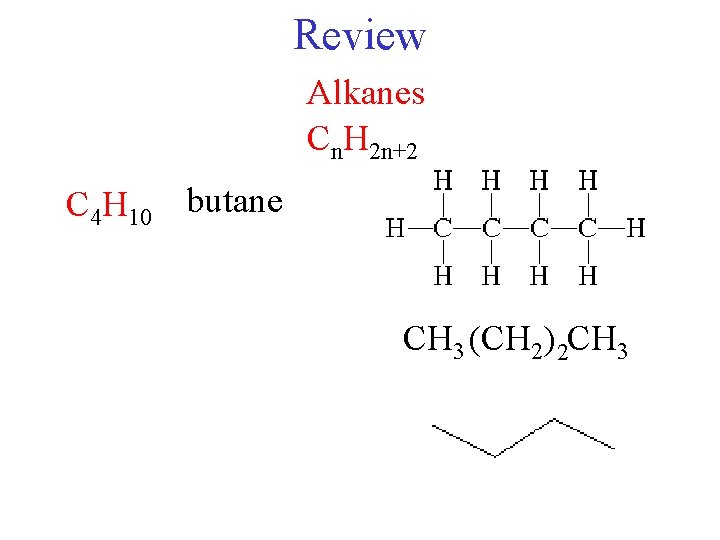
Review Alkanes Cn. H 2 n+2 butane C 4 H 10 a) ethane b) propane c) butane d) pentane CH 3 (CH 2) 2 CH 3

Newman projections butane 1 2 3 4 staggered stable eclipsed
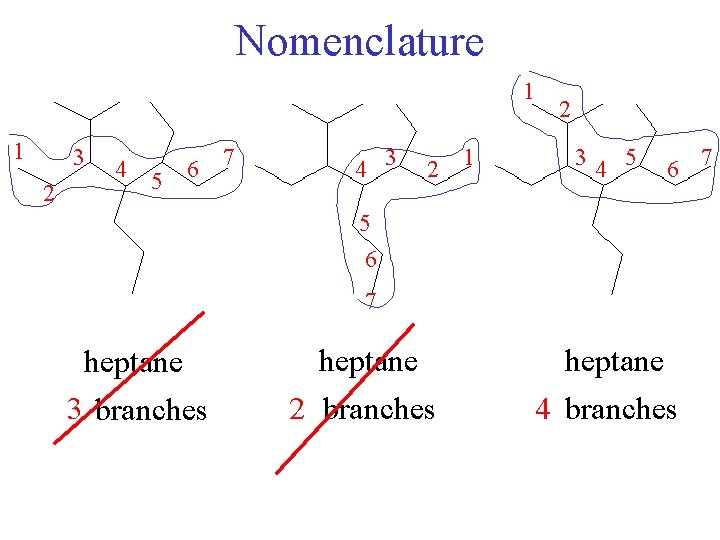
Nomenclature 1 1 3 2 4 5 6 7 4 3 2 1 2 34 5 6 7 heptane 3 branches heptane 2 branches heptane 4 branches
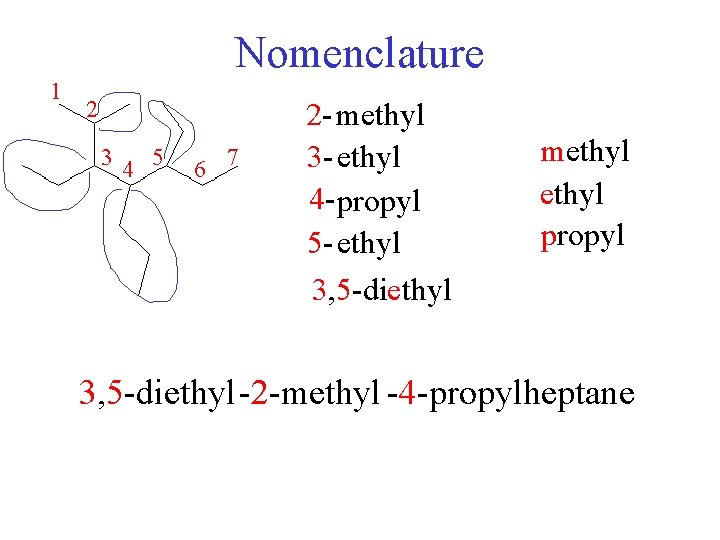
Nomenclature 1 2 34 5 6 7 2 - methyl 3 - ethyl 4 - propyl 5 - ethyl 3, 5 -diethyl methyl propyl 3, 5 -diethyl-2 -methyl -4 -propylheptane
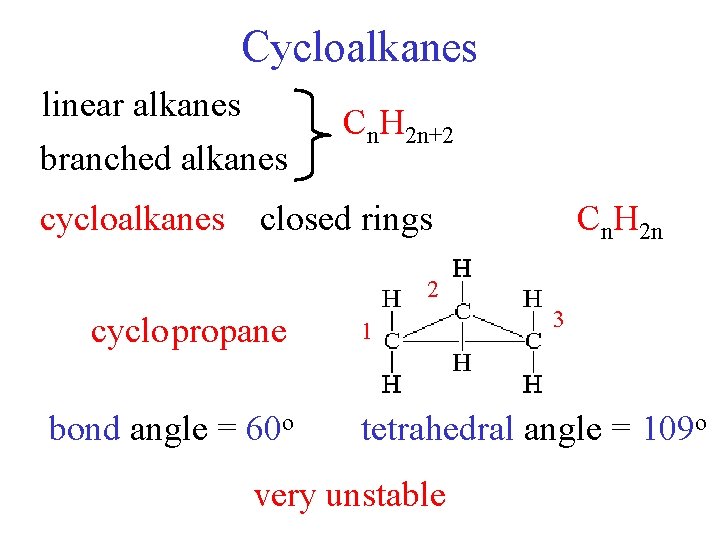
Cycloalkanes linear alkanes branched alkanes Cn. H 2 n+2 cycloalkanes closed rings Cn. H 2 n 2 cyclo propane bond angle = 60 o 1 3 tetrahedral angle = 109 o very unstable

Cycloalkanes cyclobutane bond angle = 88 o tetrahedral angle = 109 o unstable
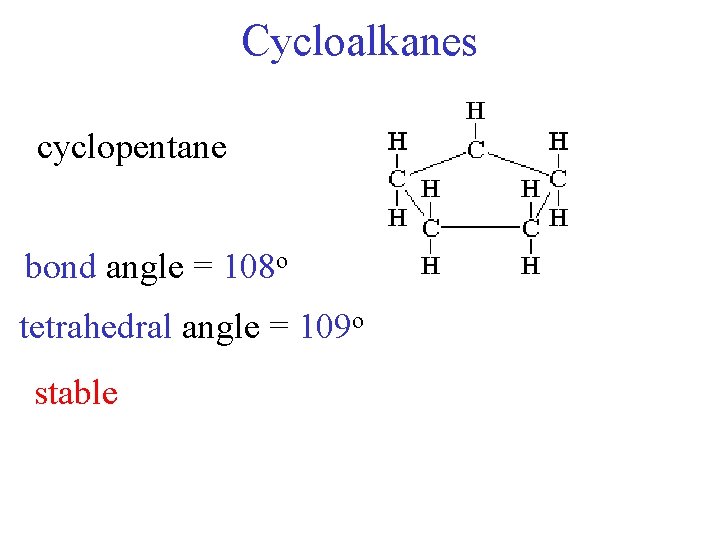
Cycloalkanes cyclopentane bond angle = 108 o tetrahedral angle = 109 o stable
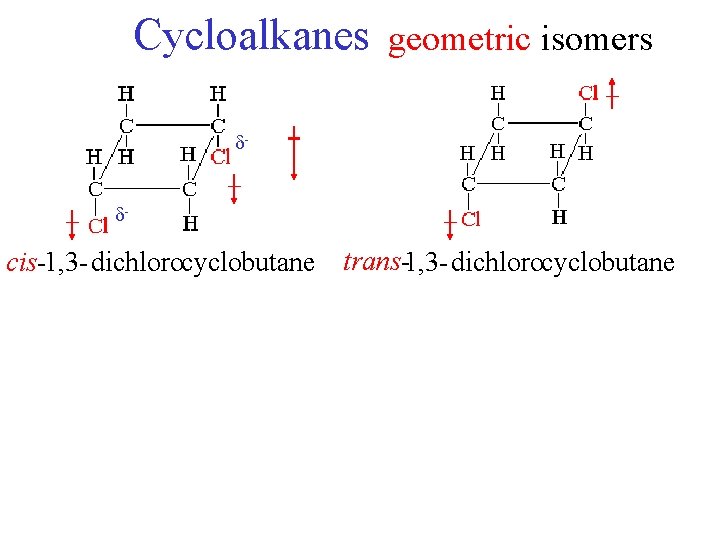
Cycloalkanes geometric isomers - cis-1, 3 -dichlorocyclobutane trans-1, 3 -dichlorocyclobutane
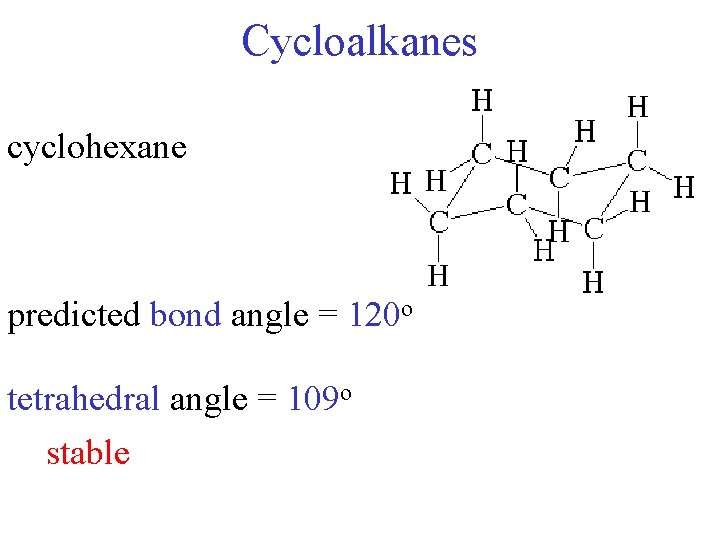
Cycloalkanes cyclohexane predicted bond angle = 120 o tetrahedral angle = 109 o stable
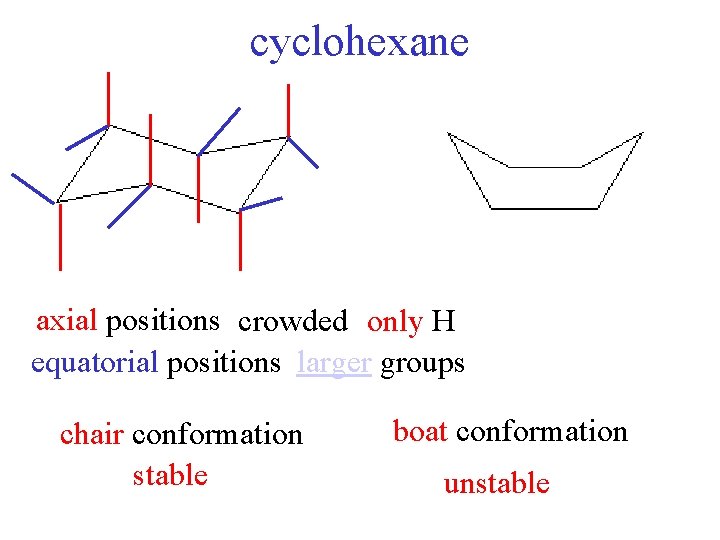
cyclohexane axial positions crowded only H equatorial positions larger groups chair conformation stable boat conformation unstable

cyclohexane cis-1, 2 -dimethylcyclohexane axial-equatorial trans-1, 2 -dimethylcyclohexane equatorial-equatorial axial-axial

Optical isomerism * C* = Stereocenter 4 different substituents bromochloroiodomethane * 3 -methylhexane * * bromocyclopentane no C* trans-1, 3 -dibromocyclopentane

Alkane Summary 1. Alkanes - sp 3 hybridized 2. Relatively unreactive Substitution with halogens Combustion 3. Non-polar IMF = London Dispersion Forces size structure

4. Free rotation around C-C bonds conformations 5. Non-cyclic alkanes - structural isomers 6. Cyclic alkanes - geometric isomers cis-, trans 7. Alkanes - optical isomers stereocenters C*
- Slides: 14