Review 4 Chapter 17 18 Thermodynamics and Electrochemistry


- Slides: 6

Review 4 Chapter 17 -18 Thermodynamics and Electrochemistry

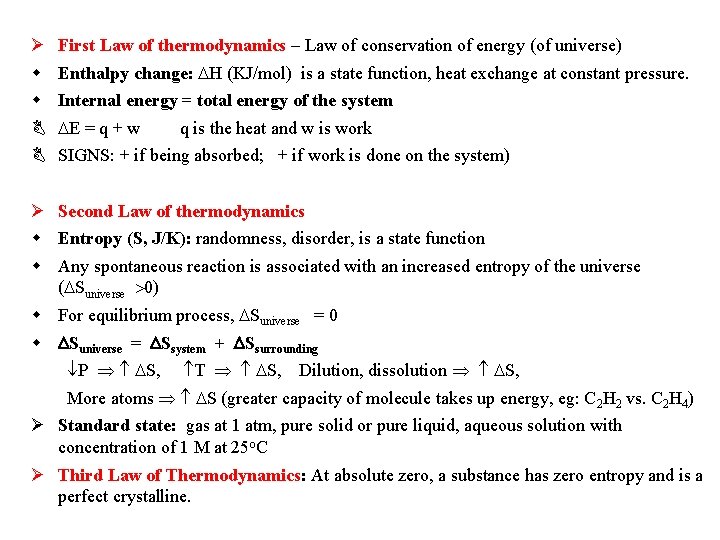
First Law of thermodynamics – Law of conservation of energy (of universe) Enthalpy change: H (KJ/mol) is a state function, heat exchange at constant pressure. Internal energy = total energy of the system E = q + w q is the heat and w is work SIGNS: + if being absorbed; + if work is done on the system) Second Law of thermodynamics Entropy (S, J/K): randomness, disorder, is a state function Any spontaneous reaction is associated with an increased entropy of the universe ( Suniverse 0) For equilibrium process, Suniverse = 0 Suniverse = Ssystem + Ssurrounding P S, T S, Dilution, dissolution S, More atoms S (greater capacity of molecule takes up energy, eg: C 2 H 2 vs. C 2 H 4) Standard state: gas at 1 atm, pure solid or pure liquid, aqueous solution with concentration of 1 M at 25 o. C Third Law of Thermodynamics: At absolute zero, a substance has zero entropy and is a perfect crystalline.

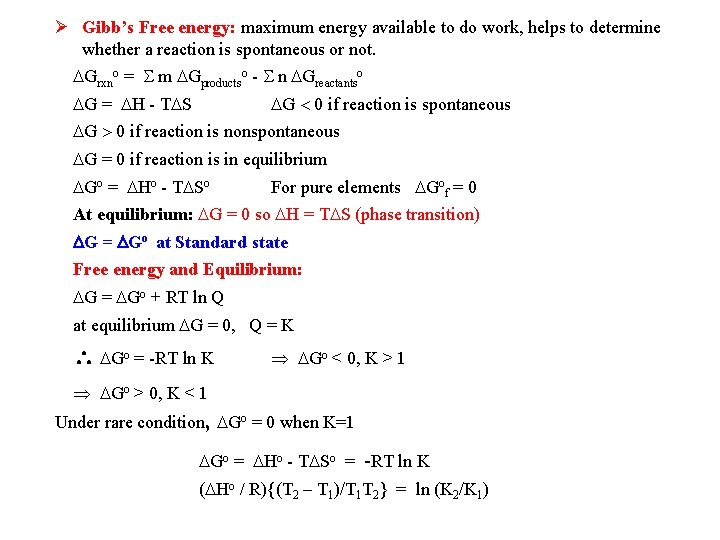
Gibb’s Free energy: maximum energy available to do work, helps to determine whether a reaction is spontaneous or not. Grxno = m Gproductso - n Greactantso G = H - T S G 0 if reaction is spontaneous G 0 if reaction is nonspontaneous G = 0 if reaction is in equilibrium Go = Ho - T So For pure elements Gof = 0 At equilibrium: G = 0 so H = T S (phase transition) G = Go at Standard state Free energy and Equilibrium: G = Go + RT ln Q at equilibrium G = 0, Q = K Go = -RT ln K Go < 0, K > 1 Go > 0, K < 1 Under rare condition, Go = 0 when K=1 Go = Ho - T So = -RT ln K ( Ho / R){(T 2 – T 1)/T 1 T 2} = ln (K 2/K 1)

Chapter 18: Electrochemistry (Chang – Chap 19) • Redox reactions: • Oxidation states: page 175 -181, section 4. 9 -4. 10. See Chapter 4 • Oxidation: ON, lose electrons, reducing agent • Reduction: ON, gain electrons, oxidizing agent Balance redox reaction: • Write down half-reactions (oxidation & reduction) • Balance all elements except Oxygen and Hydrogen • Use H 2 O balance oxygen • Use H+ balance hydrogen • Balance charges with electrons • Make sure both reduction half and oxidation half have the same number of electrons • Combine two half reactions • Use OH- to balance out the H+ if the solution is basic

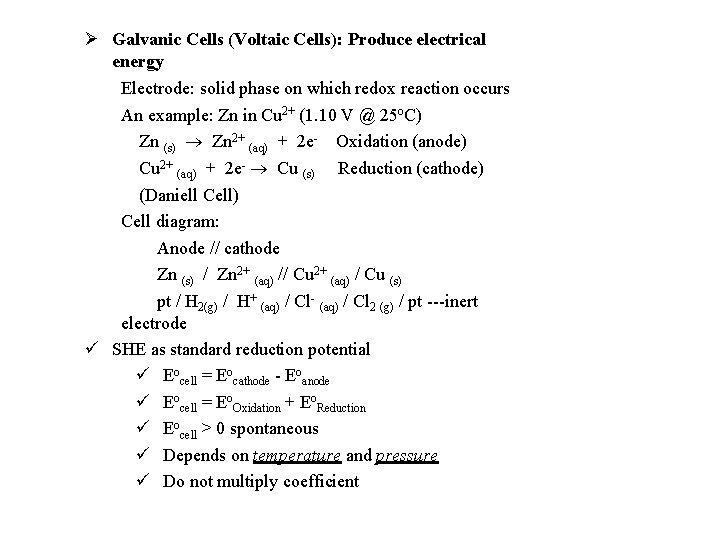
Galvanic Cells (Voltaic Cells): Produce electrical energy Electrode: solid phase on which redox reaction occurs An example: Zn in Cu 2+ (1. 10 V @ 25 o. C) Zn (s) Zn 2+ (aq) + 2 e- Oxidation (anode) Cu 2+ (aq) + 2 e- Cu (s) Reduction (cathode) (Daniell Cell) Cell diagram: Anode // cathode Zn (s) / Zn 2+ (aq) // Cu 2+ (aq) / Cu (s) pt / H 2(g) / H+ (aq) / Cl- (aq) / Cl 2 (g) / pt ---inert electrode SHE as standard reduction potential Eocell = Eocathode - Eoanode Eocell = Eo. Oxidation + Eo. Reduction Eocell > 0 spontaneous Depends on temperature and pressure Do not multiply coefficient

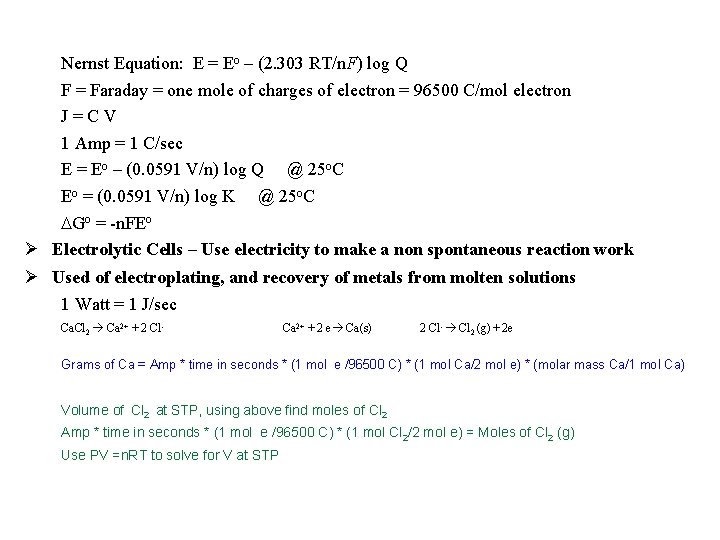
Nernst Equation: E = Eo – (2. 303 RT/n. F) log Q F = Faraday = one mole of charges of electron = 96500 C/mol electron J=CV 1 Amp = 1 C/sec E = Eo – (0. 0591 V/n) log Q @ 25 o. C Eo = (0. 0591 V/n) log K @ 25 o. C Go = -n. FEo Electrolytic Cells – Use electricity to make a non spontaneous reaction work Used of electroplating, and recovery of metals from molten solutions 1 Watt = 1 J/sec Ca. Cl 2 Ca 2+ + 2 Cl- Ca 2+ + 2 e Ca(s) 2 Cl- Cl 2 (g) + 2 e Grams of Ca = Amp * time in seconds * (1 mol e /96500 C) * (1 mol Ca/2 mol e) * (molar mass Ca/1 mol Ca) Volume of Cl 2 at STP, using above find moles of Cl 2 Amp * time in seconds * (1 mol e /96500 C) * (1 mol Cl 2/2 mol e) = Moles of Cl 2 (g) Use PV =n. RT to solve for V at STP