Reversible Reactions and Equilbrium PrenticeHall Chapter 18 2

Reversible Reactions and Equilbrium Prentice-Hall Chapter 18. 2

Objectives v Describe how the amounts of reactants and products change in a chemical system at equilibrium. v Identify three stresses that can change the equilibrium position of a chemical system. v Explain what the value of Keq the position of equilibrium. indicates about

In the early 1900 s, German chemists refined the process of making ammonia from elemental nitrogen and hydrogen. This process allows the manufacture of nitrogen fertilizers.

Reversible Reactions At chemical equilibrium, no net change occurs in the actual amounts of the components of the system. A reversible reaction is one in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously.

Equilibrium If the rate of the shoppers going up the escalator is equal to the rate of the shoppers going down, then the number of shoppers on each floor remains constant, and there is an equilibrium.

SO 2 and O 2 react to give SO 3 decomposes to SO 2 and O 2 At equilibrium, all three types of molecules are present.
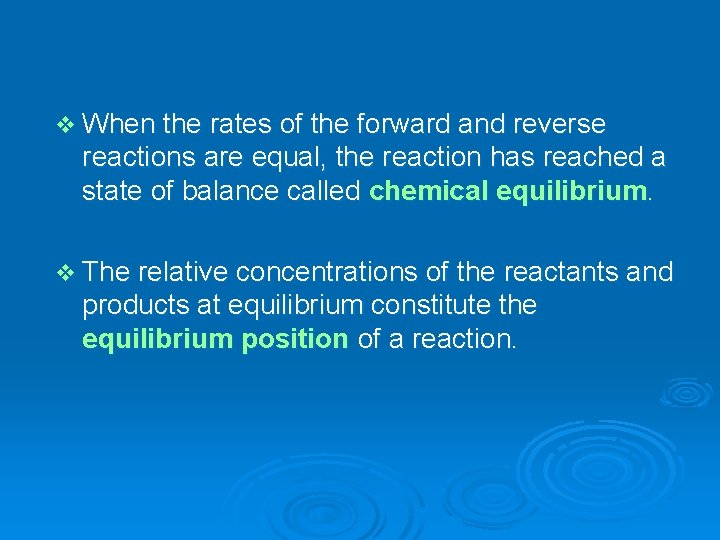
v When the rates of the forward and reverse reactions are equal, the reaction has reached a state of balance called chemical equilibrium. v The relative concentrations of the reactants and products at equilibrium constitute the equilibrium position of a reaction.

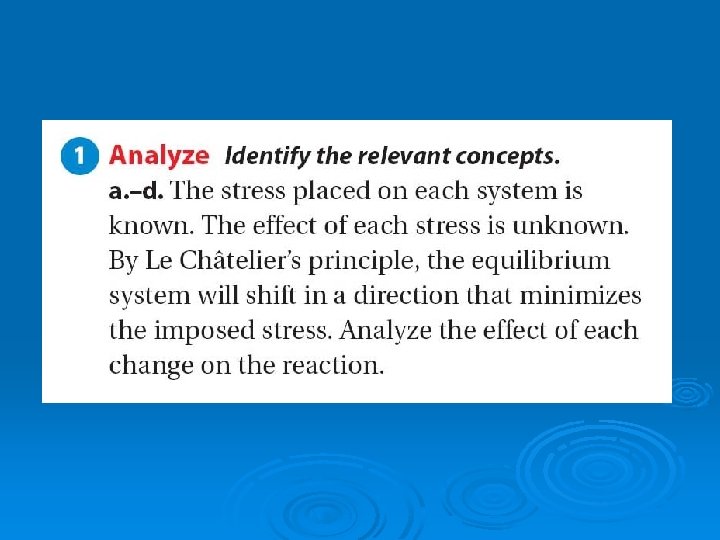
Le Châtelier’s Principle The French chemist Le Châtelier proposed what has come to be called: Le Châtelier’s principle: If a stress is applied to a system in dynamic equilibrium, the system changes in a way that relieves the stress.
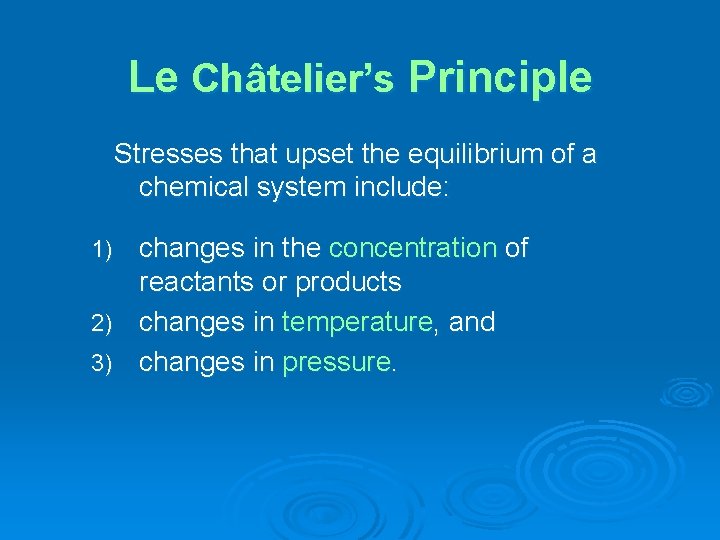
Le Châtelier’s Principle Stresses that upset the equilibrium of a chemical system include: changes in the concentration of reactants or products 2) changes in temperature, and 3) changes in pressure. 1)

Concentration Rapid breathing during and after vigorous exercise helps reestablish the body’s correct CO 2: H 2 CO 3 equilibrium, keeping the acid concentration in the blood within a safe range.
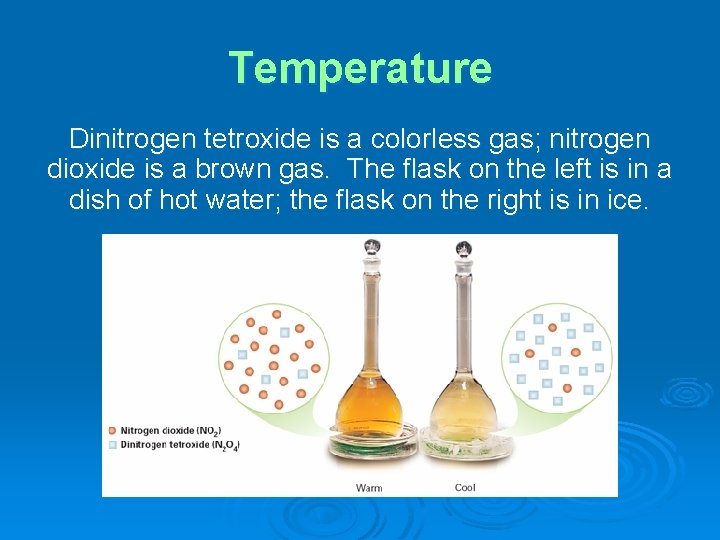
Temperature Dinitrogen tetroxide is a colorless gas; nitrogen dioxide is a brown gas. The flask on the left is in a dish of hot water; the flask on the right is in ice.

Pressure affects a mixture of nitrogen, hydrogen, and ammonia at equilibrium.



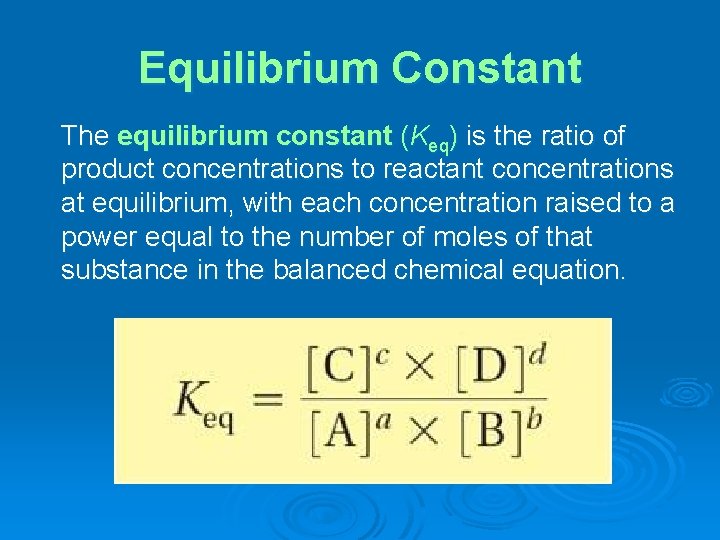
Equilibrium Constant The equilibrium constant (Keq) is the ratio of product concentrations to reactant concentrations at equilibrium, with each concentration raised to a power equal to the number of moles of that substance in the balanced chemical equation.

Equilibrium Constant A value of Keq greater than 1 means that products are favored over reactants; a value of Keq less than 1 means that reactants are favored over products. Keq > 1 products are favored at equilibrium Keq < 1 reactants are favored at equilibrium

Equilibrium Constant

Equilibrium Constant

Equilibrium Constant

Equilibrium Constant
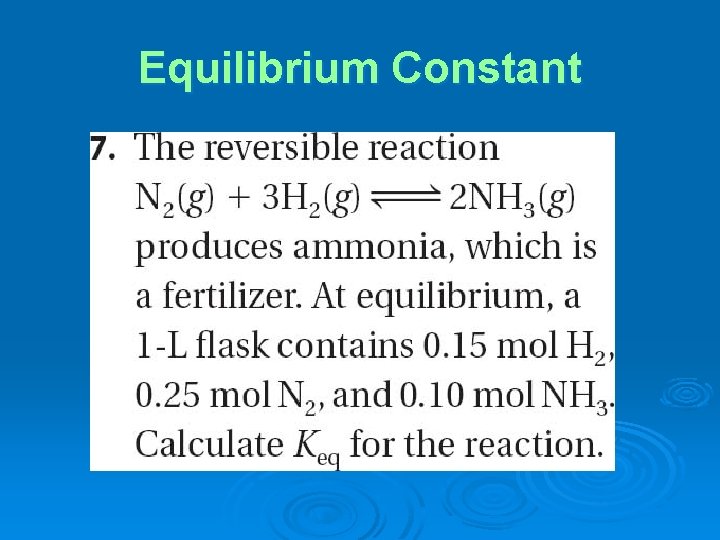
Equilibrium Constant

Equilibrium Constant

Equilibrium Constant
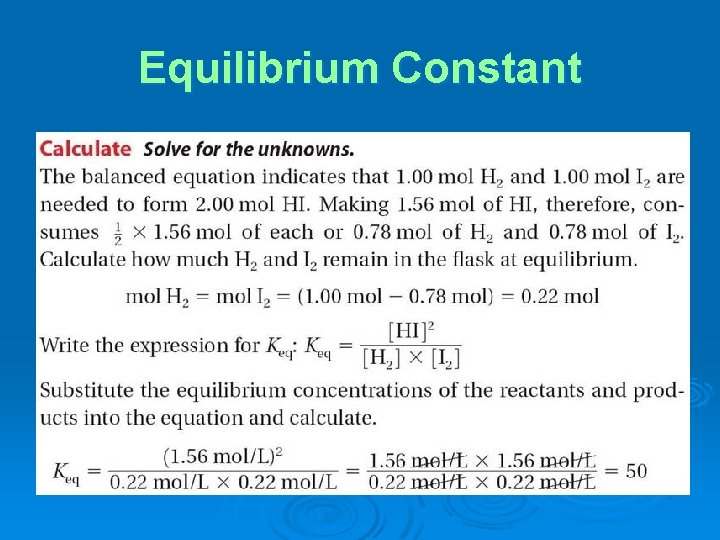
Equilibrium Constant

Equilibrium Constant

Equilibrium Constant

1. In a reaction at equilibrium, reactants and products a) decrease in concentration. b) form at equal rates. c) have equal concentrations. d) have stopped reacting.

1. In a reaction at equilibrium, reactants and products a) decrease in concentration. b) form at equal rates. c) have equal concentrations. d) have stopped reacting.

2. In the reaction 2 NO 2(g) ↔ 2 NO(g) + O 2(g), increasing the pressure on the reaction would cause a) the amount of NO to increase. b) the amount of NO 2 to increase. c) nothing to happen. d) the amount of O 2 to increase.

2. In the reaction 2 NO 2(g) ↔ 2 NO(g) + O 2(g), increasing the pressure on the reaction would cause a) the amount of NO to increase. b) the amount of NO 2 to increase. c) nothing to happen. d) the amount of O 2 to increase.

3. Changing which of the following would NOT affect the equilibrium position of a chemical reaction? a) concentration of a reactant only b) concentration of a product only c) temperature only d) volume only

3. Changing which of the following would NOT affect the equilibrium position of a chemical reaction? a) concentration of a reactant only b) concentration of a product only c) temperature only d) volume only

4. For the following reaction, Keq = 1. A(g) + B(g) ↔ C(g) + D(g) Therefore, at equilibrium a) [C] = [A]. b) [A][B] = 0. c) [AB] = [CD] = 1. d) [A][B] = [C][D].

4. For the following reaction, Keq = 1. A(g) + B(g) ↔ C(g) + D(g) Therefore, at equilibrium a) [C] = [A]. b) [A][B] = 0. c) [AB] = [CD] = 1. d) [A][B] = [C][D].
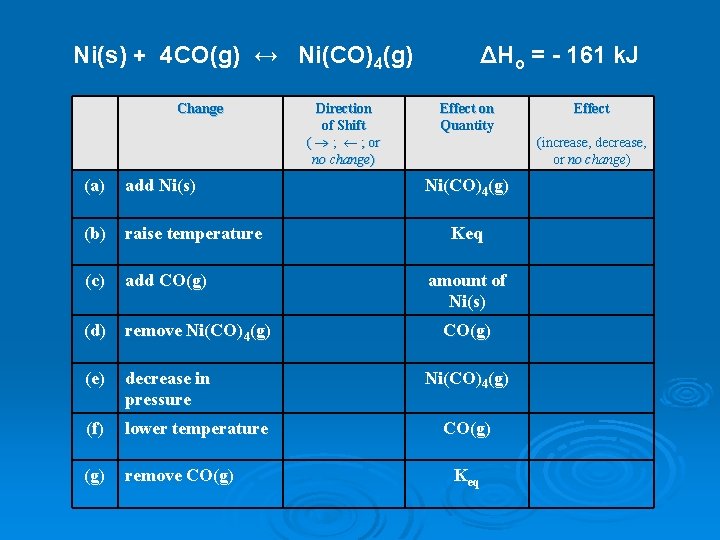
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change (a) add Ni(s) (b) raise temperature (c) add CO(g) (d) remove Ni(CO)4(g) (e) decrease in pressure (f) lower temperature (g) remove CO(g) Direction of Shift ( ® ; ¬ ; or no change) ΔHo = - 161 k. J Effect on Quantity Effect (increase, decrease, or no change) Ni(CO)4(g) Keq amount of Ni(s) CO(g) Ni(CO)4(g) CO(g) Keq
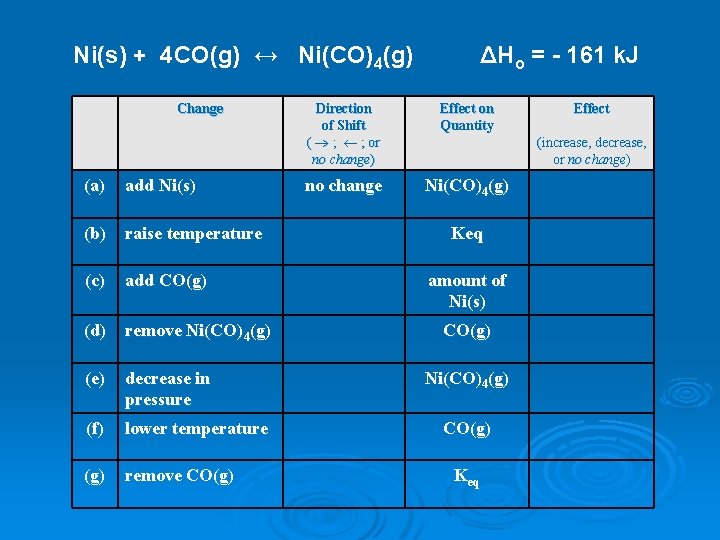
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change (a) add Ni(s) (b) raise temperature (c) add CO(g) (d) remove Ni(CO)4(g) (e) decrease in pressure (f) lower temperature (g) remove CO(g) ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity no change Ni(CO)4(g) Effect (increase, decrease, or no change) Keq amount of Ni(s) CO(g) Ni(CO)4(g) CO(g) Keq
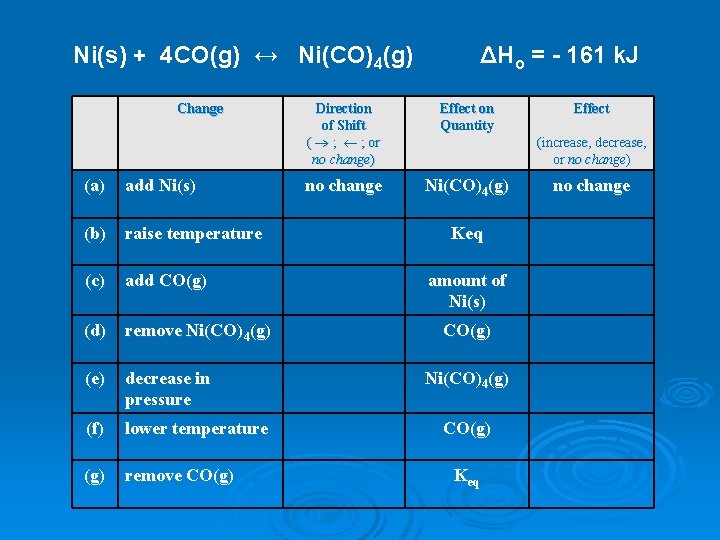
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change (a) add Ni(s) (b) raise temperature (c) add CO(g) (d) remove Ni(CO)4(g) (e) decrease in pressure (f) lower temperature (g) remove CO(g) ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity no change Ni(CO)4(g) Effect (increase, decrease, or no change) Keq amount of Ni(s) CO(g) Ni(CO)4(g) CO(g) Keq no change
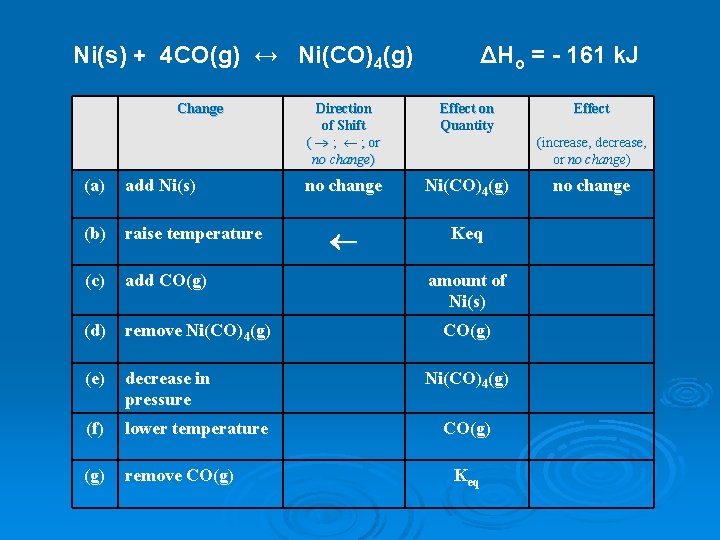
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change (a) add Ni(s) (b) raise temperature (c) add CO(g) (d) remove Ni(CO)4(g) (e) decrease in pressure (f) lower temperature (g) remove CO(g) ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity no change Ni(CO)4(g) ¬ Keq Effect (increase, decrease, or no change) amount of Ni(s) CO(g) Ni(CO)4(g) CO(g) Keq no change
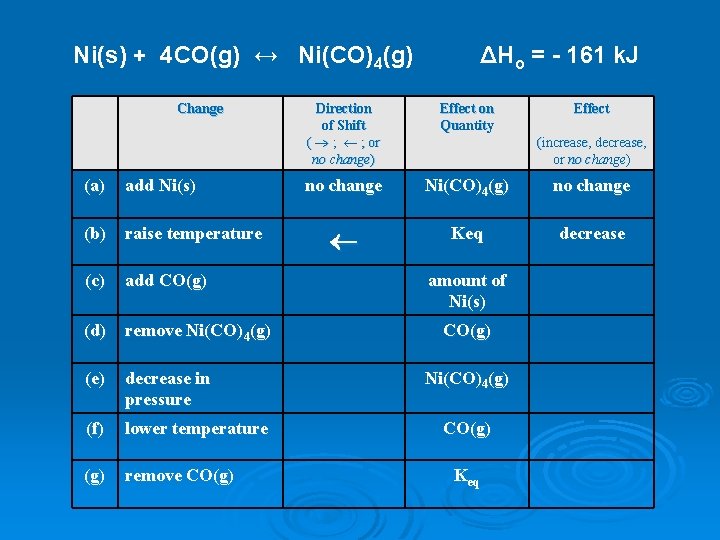
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change (a) add Ni(s) (b) raise temperature (c) add CO(g) (d) remove Ni(CO)4(g) (e) decrease in pressure (f) lower temperature (g) remove CO(g) ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change ¬ Keq decrease (increase, decrease, or no change) amount of Ni(s) CO(g) Ni(CO)4(g) CO(g) Keq
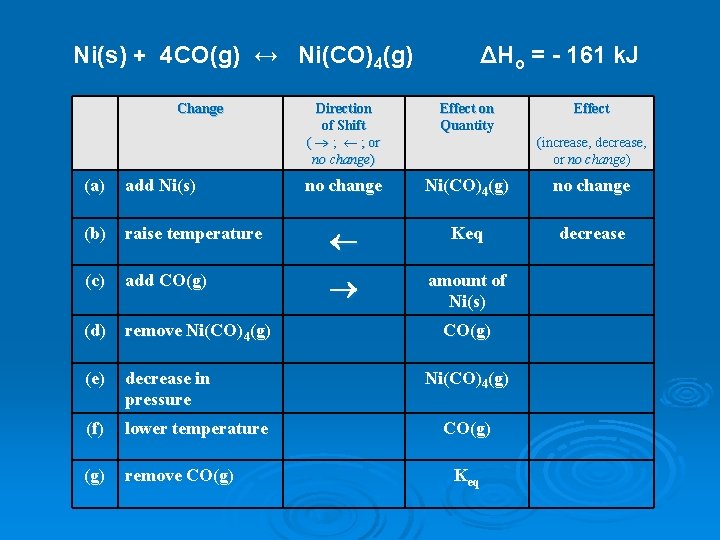
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change decrease (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq (c) add CO(g) ® amount of Ni(s) (d) remove Ni(CO)4(g) (e) decrease in pressure (f) lower temperature (g) remove CO(g) Ni(CO)4(g) CO(g) Keq
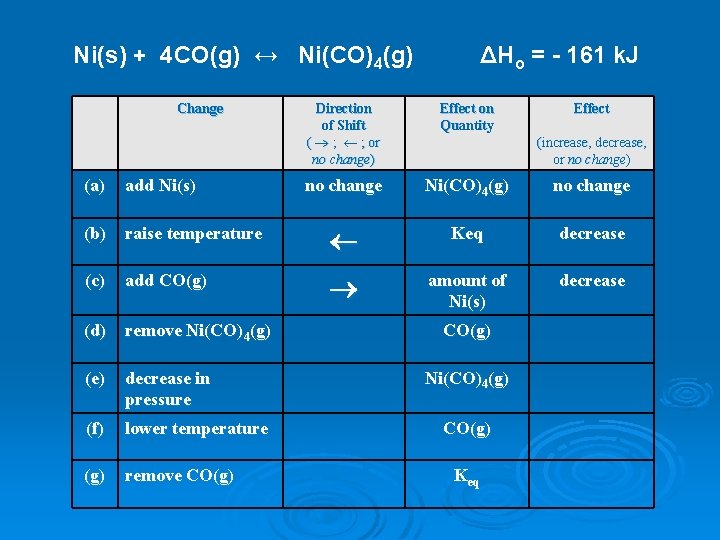
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq decrease (c) add CO(g) ® amount of Ni(s) decrease (d) remove Ni(CO)4(g) (e) decrease in pressure (f) lower temperature (g) remove CO(g) Ni(CO)4(g) CO(g) Keq
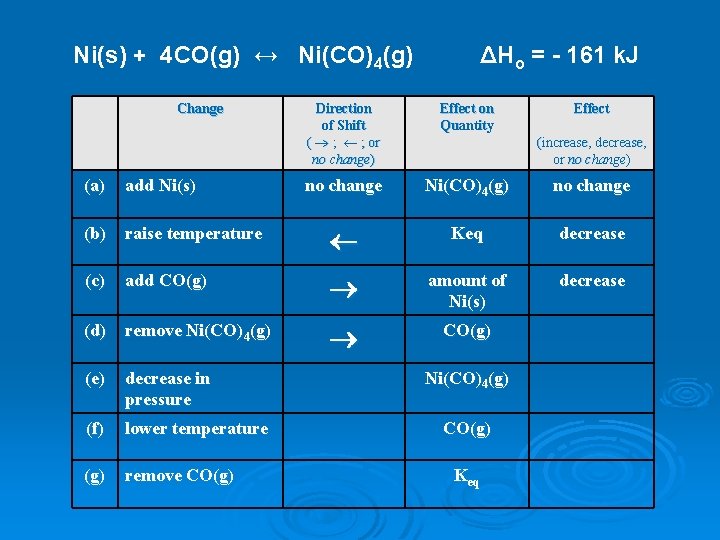
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq decrease (c) add CO(g) ® amount of Ni(s) decrease (d) remove Ni(CO)4(g) ® CO(g) (e) decrease in pressure (f) lower temperature (g) remove CO(g) Ni(CO)4(g) CO(g) Keq
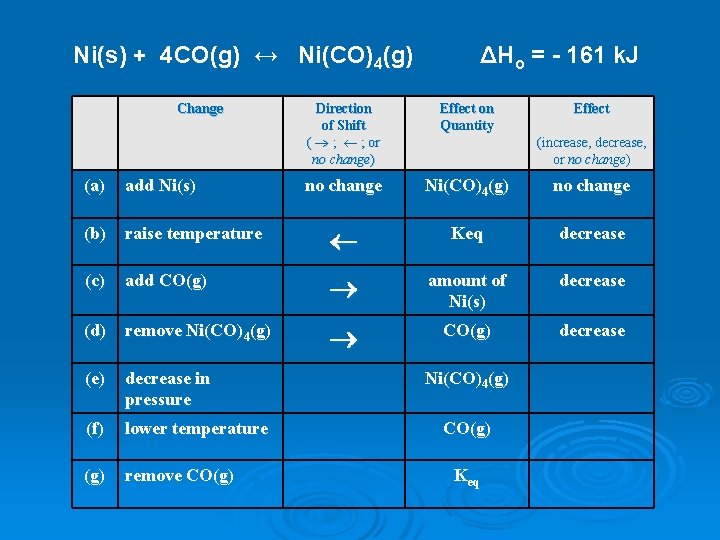
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq decrease (c) add CO(g) ® amount of Ni(s) decrease (d) remove Ni(CO)4(g) ® CO(g) decrease (e) decrease in pressure (f) lower temperature (g) remove CO(g) Ni(CO)4(g) CO(g) Keq
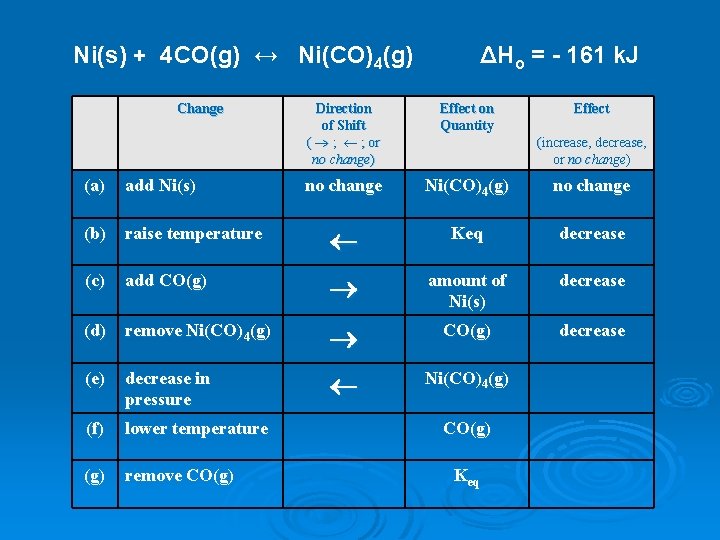
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq decrease (c) add CO(g) ® amount of Ni(s) decrease (d) remove Ni(CO)4(g) ® CO(g) decrease (e) decrease in pressure ¬ Ni(CO)4(g) (f) lower temperature (g) remove CO(g) Keq
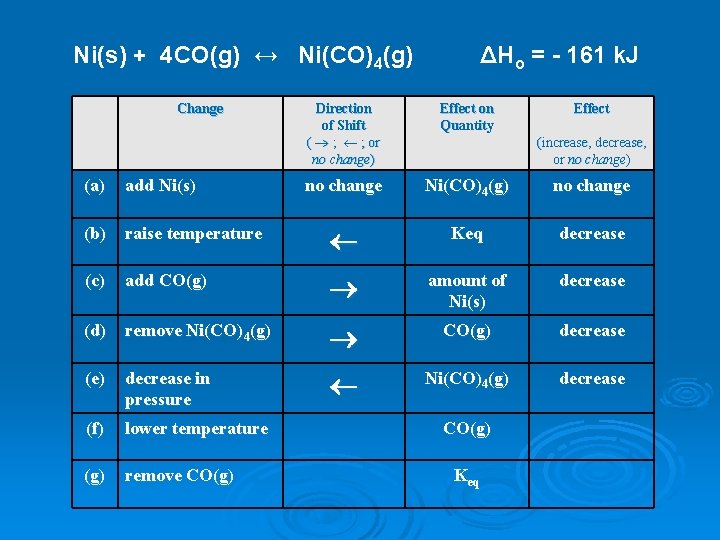
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq decrease (c) add CO(g) ® amount of Ni(s) decrease (d) remove Ni(CO)4(g) ® CO(g) decrease (e) decrease in pressure ¬ Ni(CO)4(g) decrease (f) lower temperature (g) remove CO(g) Keq
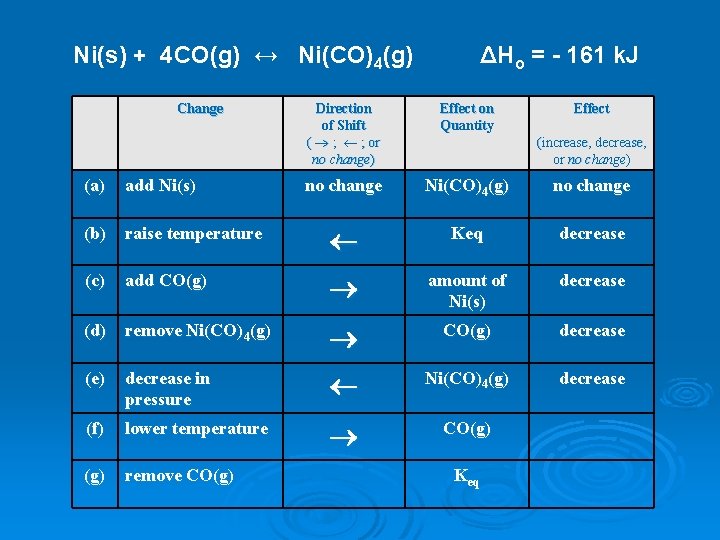
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq decrease (c) add CO(g) ® amount of Ni(s) decrease (d) remove Ni(CO)4(g) ® CO(g) decrease (e) decrease in pressure ¬ Ni(CO)4(g) decrease (f) lower temperature ® CO(g) remove CO(g) Keq
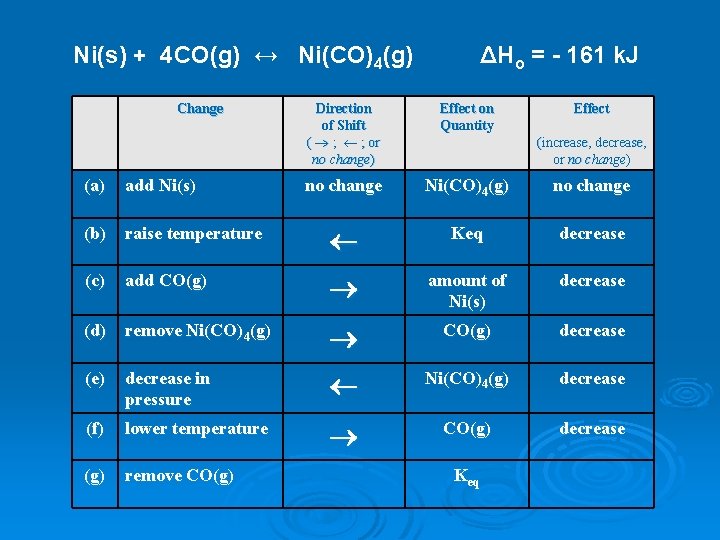
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq decrease (c) add CO(g) ® amount of Ni(s) decrease (d) remove Ni(CO)4(g) ® CO(g) decrease (e) decrease in pressure ¬ Ni(CO)4(g) decrease (f) lower temperature ® CO(g) decrease (g) remove CO(g) Keq
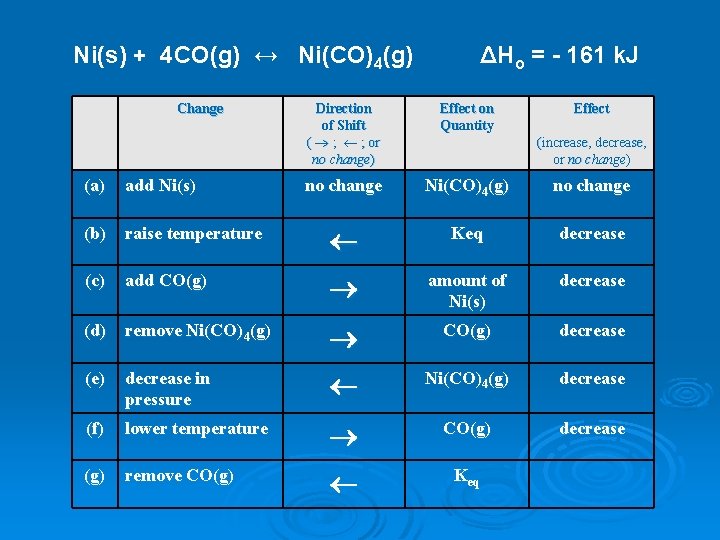
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq decrease (c) add CO(g) ® amount of Ni(s) decrease (d) remove Ni(CO)4(g) ® CO(g) decrease (e) decrease in pressure ¬ Ni(CO)4(g) decrease (f) lower temperature ® CO(g) decrease (g) remove CO(g) ¬ Keq
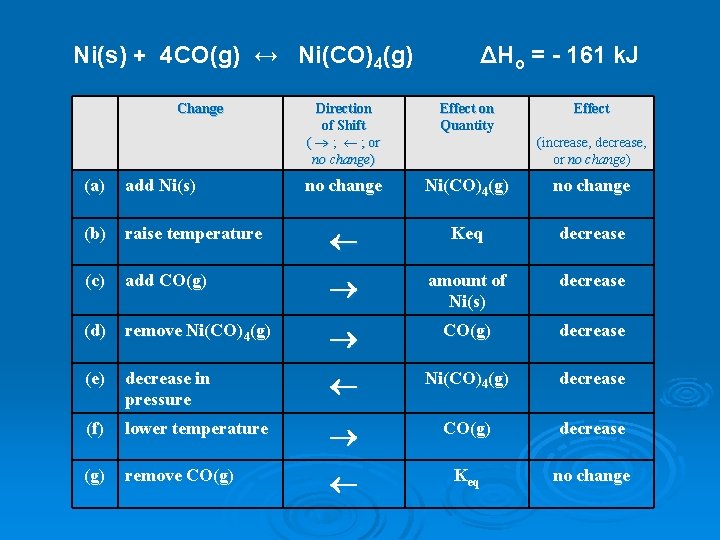
Ni(s) + 4 CO(g) ↔ Ni(CO)4(g) Change ΔHo = - 161 k. J Direction of Shift ( ® ; ¬ ; or no change) Effect on Quantity Effect no change Ni(CO)4(g) no change (increase, decrease, or no change) (a) add Ni(s) (b) raise temperature ¬ Keq decrease (c) add CO(g) ® amount of Ni(s) decrease (d) remove Ni(CO)4(g) ® CO(g) decrease (e) decrease in pressure ¬ Ni(CO)4(g) decrease (f) lower temperature ® CO(g) decrease (g) remove CO(g) ¬ Keq no change
- Slides: 52